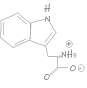
TRIM Technology –Maximize Efficiency and Precision in Library Constructions.
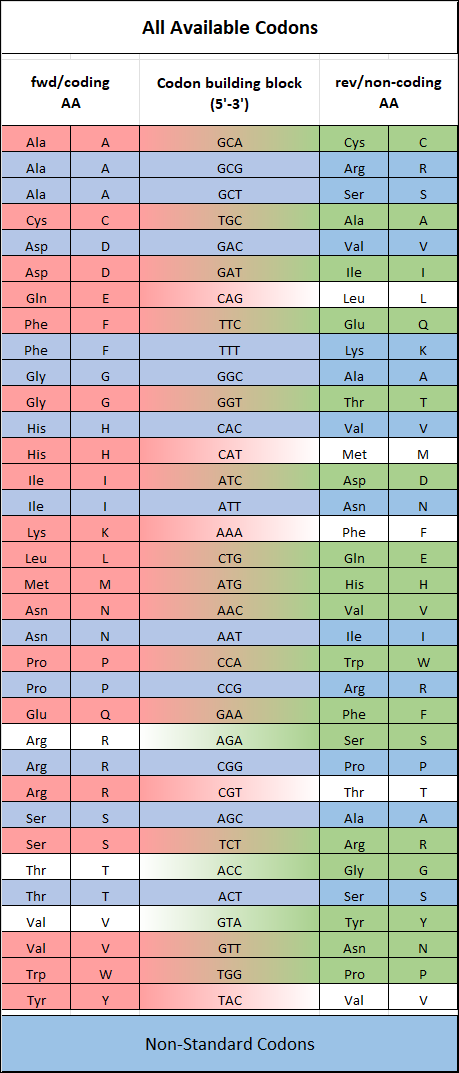
Codon Availability
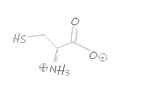
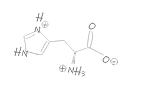
Our TRImer codon building blocks are manufactured in-house using proprietary synthesis processes. We maintain a stock of 32 of the 64 possible codon building blocks, selectively omitting those that generate stop codons or have minimal usage in target organisms.
In addition, G/C-rich codons are generally avoided in standard codon sets whenever possible, as they are more difficult to synthesize with high purity and can increase the G/C ratio in oligonucleotides, potentially leading to unwanted secondary structures. As a result, we cannot always provide identical forward (coding) and reverse (non-coding) codons, as some sequences are intentionally excluded to optimize quality and performance.
On request we may adjust the codons based on your wishes and the available codons.
Codons may also be adjusted for avoiding restriction sites.
Standard Codons
For convenience and optimization, we use one set of codons for most of our TRIM mixes. (see table below)
These codons have been chosen as an optimized hybrid set for E. coli and H. sapiens. (see Codon usage)
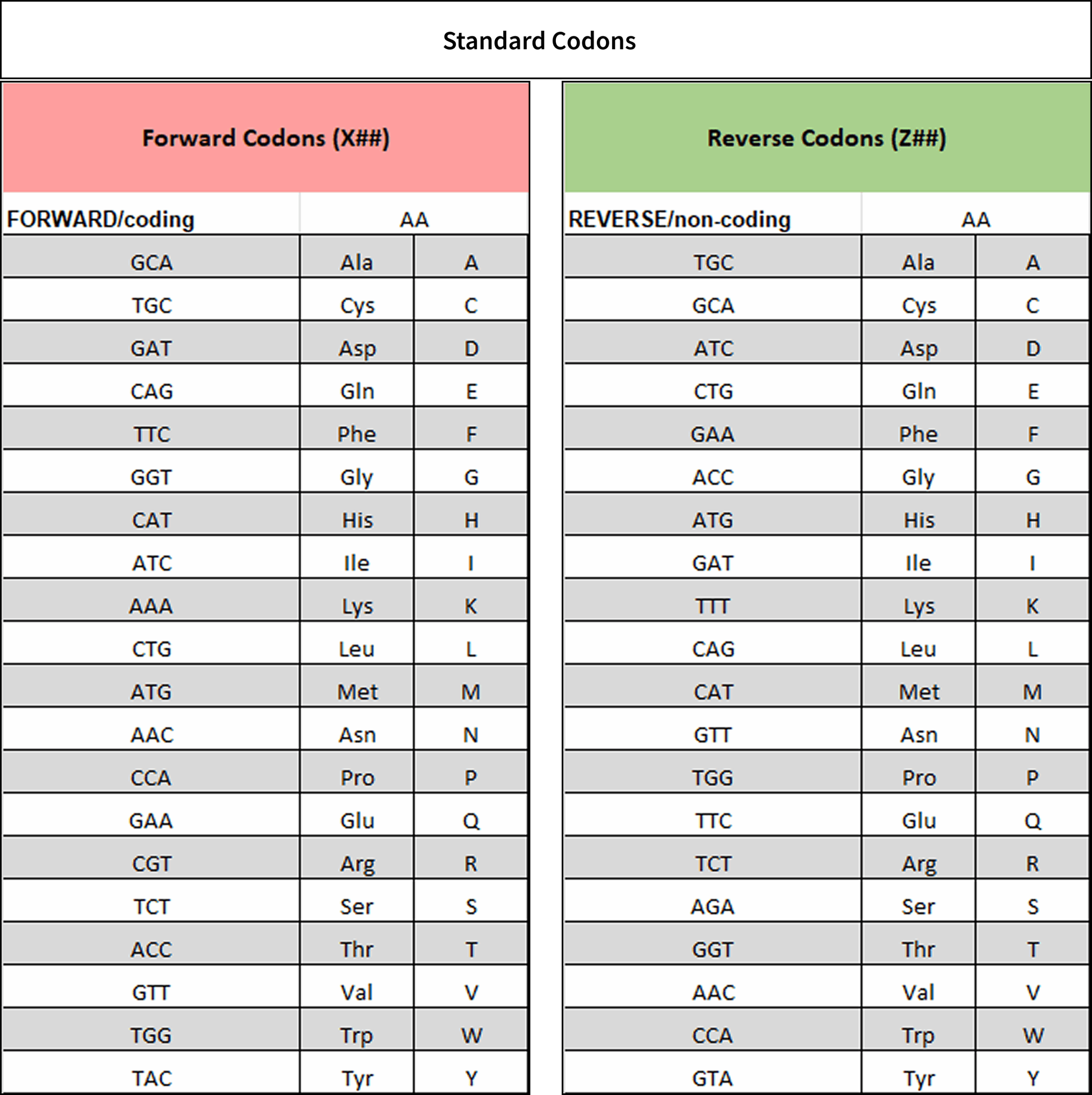
For reasons of convenient library assembly whilst keeping the oligo design as short as possible, we offer the synthesis of either forward/coding and reverse/non-coding oligos.
When designing antibody libraries using TRIM oligos, the choice between forward and reverse codons depends on the library design and the assembly strategy you choose. In solid-phase oligonucleotide synthesis, we’re limited by the length of the oligo that can be produced without sacrificing quality and introducing mutations. After a certain length, with every new nucleotide introduced, the error rate grows. Sometimes, the desired length is too long to produce as a single oligonucleotide, in which case smaller oligo fragments must be assembled to build a longer sequence.
This can be achieved by assembling shorter oligos together in one of two ways: 1) by ligating two sense oligos with a splint oligo (that has complementary overhangs for both sequences), or 2) by running a PCR using a longer antisense primer with an overhang for the sense target sequence and a longer tail that will extend the final product.
Depending on the design of the insert, it may be necessary to introduce degenerate TRImer sites into different parts of the final sequence, which requires the assembly of two TRImers. For the 1) ligation method, two sense TRImers should be ligated together, while for the 2) PCR method, a sense and an antisense TRImer oligos with a complementary overhang should be used to extend the double-stranded insert sequence.
In the instance of reverse oligos, the reverse complement of the codons will indicate how they are translated. Each codon corresponds to a specific amino acid in both the forward and reverse orientations, resulting in the distinction between forward/coding codons and reverse/non-coding codons.
For example, the sequence TTT in a forward oligo is equivalent to Lysine (K), whereas TTT in a reverse oligo codes for phenylalanine (F).
To understand why it is better to keep the oligo design as short as possible while designing the library, please look at our Resource page on the topics Oligo Length and Scale And Yield.
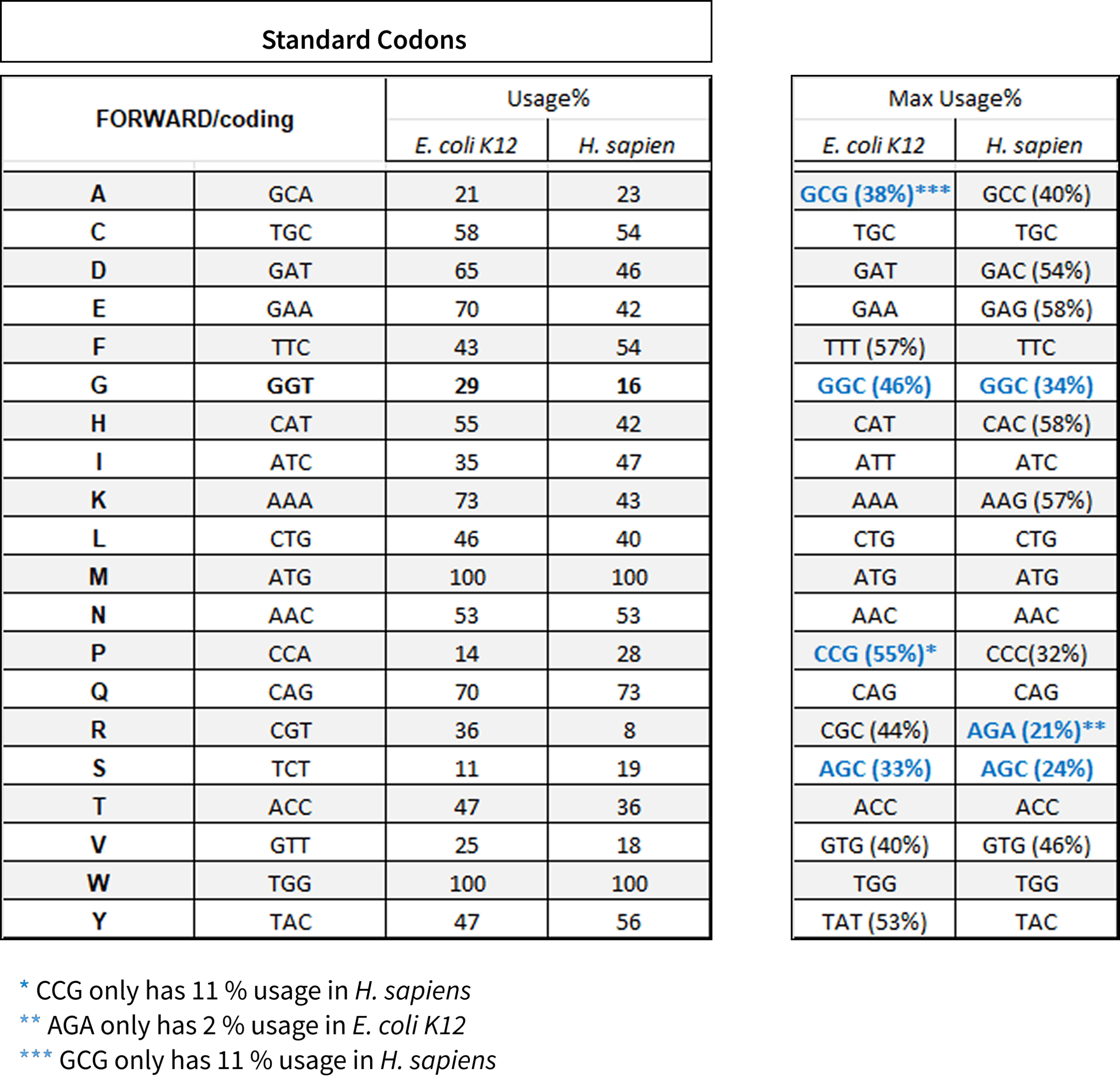
Codon Usage
The Standard Codon Set is commonly used to express human genes in E. coli to improve protein production in research. It serves as the basis for codon optimization strategies to improve expression in different organisms and aids in synthetic gene design for both E. coli and human cells.
These sets provide a useful starting point for gene design and optimization, but it’s important to consider its limitations. For optimal results, codon usage often needs to be fine-tuned based on the specific gene, expression system, and desired outcome, balancing factors such as GC content, tRNA availability, and potential effects on protein folding.
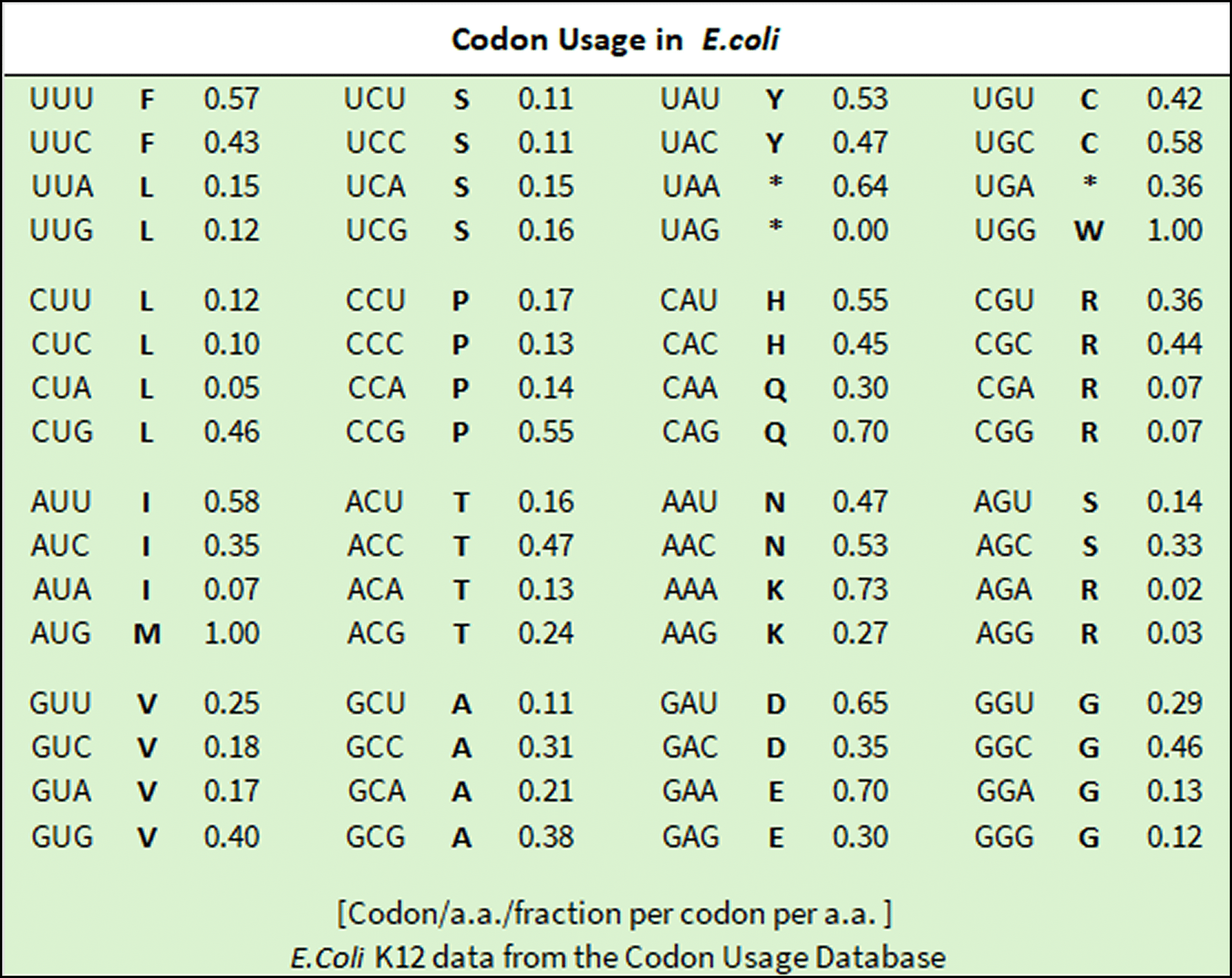
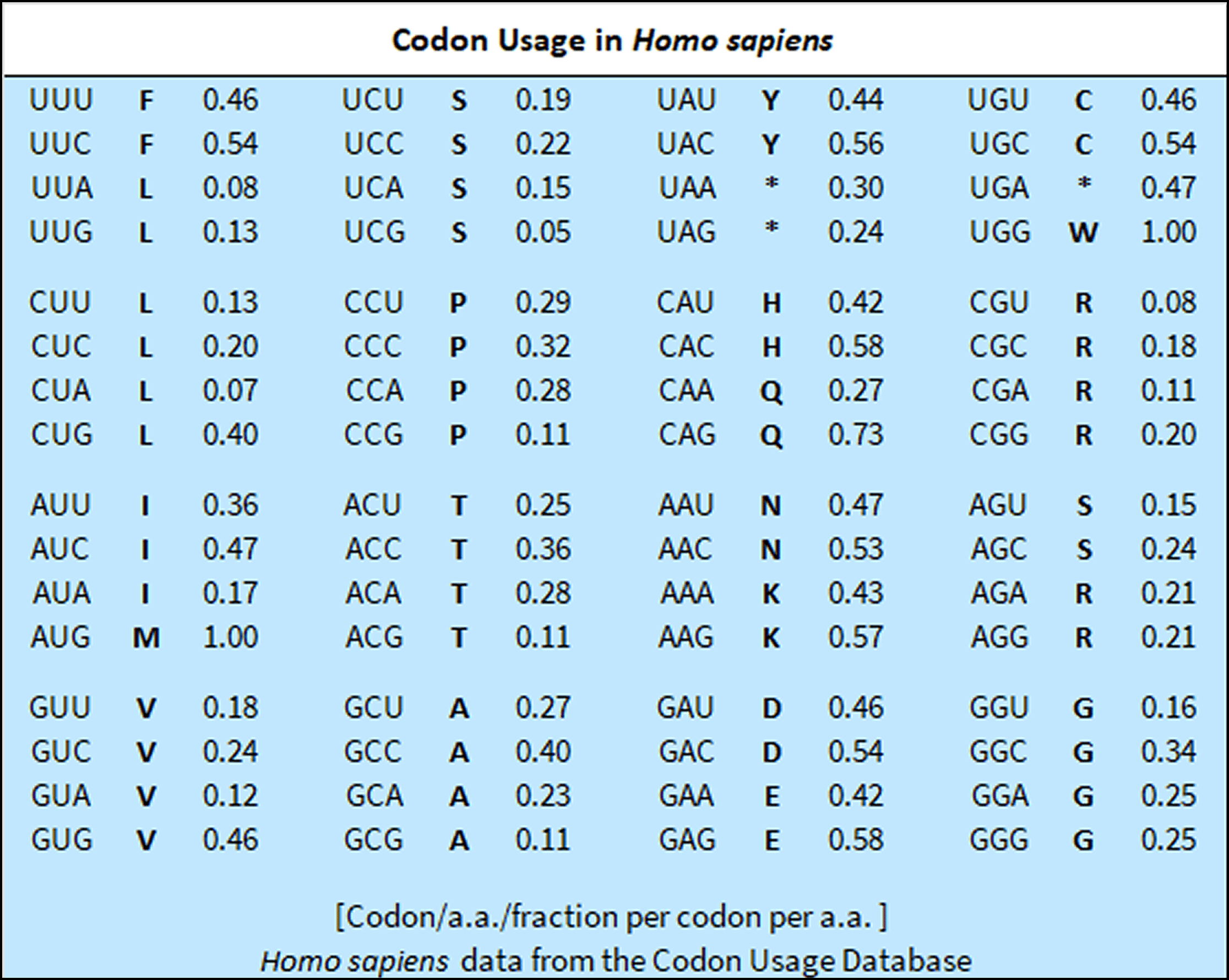
Codon origin and quality
Our TRImer codon building blocks are manufactured in-house by a team of experienced organic chemists using the most up-to-date equipment and proprietary synthesis processes. This in-house production capability allows us to maintain strict quality control using a range of validated analytical methods to ensure the highest purity and performance of our materials.
In addition, by directly managing production and inventory, we can optimize stock levels, enabling faster turnaround times and minimizing delays – ensuring that your critical research projects stay on schedule.
We are hiring
Are you ready to combine science with creativity? At Ella Biotech, you’ll join a close-knit team dedicated to advancing the life sciences. You’ll find a vibrant, respectful workplace where teamwork drives innovation and fun is always part of the mix.
Bring your talents to our team where every day is an exciting opportunity to make a difference in biotechnology and advance discovery. Let’s create the building blocks of tomorrow – together!
Contact us
„*“ zeigt erforderliche Felder an
