
Therapeutic Oligos
siRNA
Small interfering RNA (siRNA) silences specific genes by targeting complementary mRNA for degradation, making it a powerful tool in research and therapeutics for diseases such as cancer and genetic disorders, as well as in functional genomics to study gene function, pathway analysis, and large-scale screening experiments.
Synthesized as two complementary strands of RNA, siRNA often includes chemical modifications such as 2′-O-methyl groups or phosphorothioate backbones to increase stability, reduce off-target effects, and improve nuclease resistance. We use high purity methods and rigorous quality control to ensure the reliable performance of siRNA in various applications.
Length
We offer custom siRNA oligo synthesis, from 7 to 35 nucleotides, with options for chemical modifications followed by optimal purification steps to enhance stability and specificity for RNA interference applications.
Scales and Yields
The yield of oligo synthesis for siRNA is dependent on the purification method, synthesis scale, and modification complexity. Purification options like HPLC ensure the highest quality oligos, but this process will result in a reduced yield.
Please inquire if you have any specific requests on scale or yield.
siRNA
Purification Method
Scale [nmol]
Minimum Yield in nmol - Based On Oligo Length [nt]
- 10
- 15
- 20
- 25
- 30
- 35
HPLC
+ NaAc precipitation
200
25
For further information, please refer to Oligo Length, Quality and Yield as well as Scales and Yields in our Resource section.
Modifications
At Ella Biotech we provides a variety of oligo modification options for ASOs, enabling enhanced stability, specificity, and cellular uptake. Most of our standard modifications are also available for siRNA.
Please refer to the Modification Options for siRNA page for a list of the most commonly used modifications.
For specialized requirements, please contact us to discuss customized modifications that can enhance cellular delivery while optimizing siRNA performance in your research.
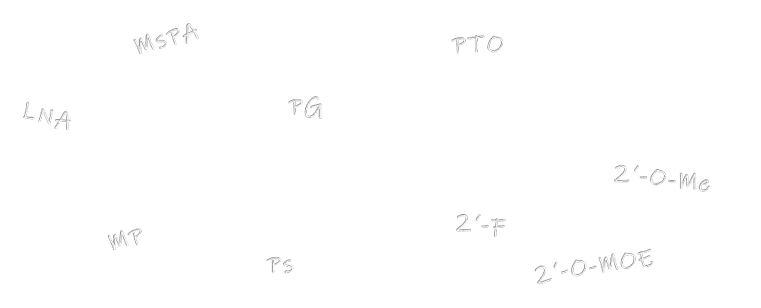
Backbone Modifications And Directionality
Backbone Modifications
siRNAs are often chemically modified to increase their stability and therapeutic efficacy. The following backbone and sugar modifications are available for our custom siRNA oligos:
Sugar Modification Option
Backbone Modification Option
DNA (A/G/C/T)
Phosphodiester (PO), Phosphorothioate (PTO/PS),
Methyl- phosphonate, Mesyl-phosphonates (µ/MsPA)
RNA (rA/rG/rC/rU)
Phosphodiester (PO), Phosphorothioate (PTO/PS),
Mesyl-phosphonates (µ/MsPA )
2’O-Methyl-RNA (oA/oG/oC/o(mC)/oU)
Phosphodiester (PO), Phosphorothioate (PTO/PS),
2’O-Methoxyethyl-RNA (eA/eG/e(mC)/eT)
Phosphodiester (PO), Phosphorothioate (PTO/PS),
2’Fluoro-RNA (fA/fG/fC/fU)
Phosphodiester (PO), Phosphorothioate (PTO/PS),
LNA bases (+A/+(mC)/+G/+T)
Phosphodiester (PO), Phosphorothioate (PS/PTO),
Mesyl- phosphonates (µ/MsPA)
Directionality
Regular phosphoramidite based oligo synthesis is done in 3′ -> 5′ direction. Change of synthesis direction offers additional functionality and enables synthetic pathways to modifications otherwise inaccessible.
3' -> 5'
5' -> 3'
DNA (A/G/C/T)
reverse DNA (A/G/C/T)
RNA (rA/rG/rC/rU)
reverse RNA (rA/rG/rC/rU)
2’O-Methyl-RNA (oA/oG/oC/oU)
On request
2’O-Methoxyethyl-RNA (eA/eG/e(mC)/eT)
On request
2’Fluoro-RNA (fA/fG/fC/fU)
On request
LNA bases (+A/+(mC)/+G/+T)
reverse LNA (+A/+mC/+G/+T)
Please inquire if you need a modification that is not part of our standard offerings.
Purification
You can order siRNA with any of our available purification options. Please note that higher purification levels improve quality but will result in lower yield, especially for longer oligos. For optimal performance, we recommend HPLC purification, as it effectively removes sulfurization byproducts and ensures high-purity oligos.
Purification Methods
Description
Desalting
Removal of truncates <6 nt and chemical residues
RP-HPLC
Removal of deletions and contaminants utilizing oligo hydrophobicity
Duplex HPLC
2nd HPLC of the hybridized oligos for removal of excess single strands after annealing
For additional details, please refer to Purification Method in the Resources section.
If you require alternative purification options, please contact us.
Delivery Form and Packaging
We offer oligonucleotides in a variety of packaging formats, including single tubes, multi-well plates, as well as bulk options, in a range of concentrations, solutions, and aliquot options. Custom vial labeling is also available upon request.
Concentration
Lyophilized or select a value from 5 µM to 5mM* (on special request)
Available Solvents
Ambion™ Nuclease Free Water (NFW), TE (10:1), low TE (10:0.1, low EDTA), D-PBS (pH 7.4)
Packaging
2-mL-tubes (standard), for larger amounts 5-mL-tubes and even 15/50 mL centrifuge tubes are possible
Aliquoting
Aliquoting to your required volumes and concentrations is available on request. This ensures accurate and convenient sample management
QC Aliquot
A small QC aliquot can be provided, ensuring product integrity and allowing performance testing without opening the main batch, streamlining reporting
* Possible concentration may depend on scale/yield, as low yields at high concentrations result in extremely small volumes that are very difficult to handle, e.g. 5 nmol at 5 mM would result in a volume of 1 µL.
Quality and Quality Control (QC)
For siRNA oligo synthesis, we ensure rigorous quality control through ESI-MS and HPLC to confirm purity and sequence integrity.
Each strand of duplex siRNA undergoes individual QC with ESI-MS and HPLC, followed by duplex QC with HPLC to verify accurate hybridization and product quality.
PTO is one of the most common modification options, where the introduction of non-bridging sulfur into the phosphodiester linkage improves nuclease resistance, biodistribution, and pharmacokinetics. However, this modification can create a chiral center that complicates quality control and purification.
Different conformations interact differently with RP-HPLC columns, causing the unified product peak to split into overlapping signals. As a result, broadened oligo peaks can interfere with n-1/n+1 species, reducing the effectiveness of RP-HPLC for purification and purity assessment.
To address these challenges while keeping screening oligo production cost-effective, we perform thorough QC via ESI-MS instead of analytical HPLC. This approach provides a comprehensive assessment of oligo quality and a clear visualization of impurity profiles. As a result, our QC criteria are primarily based on ESI-MS data, ensuring reliable analysis despite the complexities introduced by sulfur modification.
Additional Services
Additional Service Option
Description
Prepaid Oligo Service
You can set up a credit account to make your purchases easier by paying by direct debit from your prepaid credit account.
Endotoxin free production
Production of oligonucleotides on a different production line with a massively reduced level of endotoxin
Endotoxin Level (LAL)
Fast and reliable measurement of a pre-defined level of endotoxin using Charles River’s Endosafe® Nextgen-pts
Duplex Setup
Annealing of complementary oligos including ssDNA/RNA and dsDNA/RNA QC (HPLC/ESI-MS).
QC documentation
Receive specific QC data along with the oligo(s)
Documentation
Description
ESI-MS
ESI-MS spectra
ESI-MS Interpretation
ESI-MS spectra including interpretation of the signals and an ESI-MS based approximation of the purity in %.
HPLC
Analytical RP- or IEX-HPLC spectra
Duplex QC
ESI-MS and analytical HPLC of the ssDNA/RNA oligos and analytical HPLC of the Duplex
Production Time and Shipment
siRNA oligo production time varies with modification complexity, yield requirements, and order volume. Oligos with complex modifications take longer, and higher yields or larger orders may also extend production time.
The typical production time for siRNA oligo synthesis is 10 days (ranging from 7 to 15 working days) for each 96-well plate synthesis. Similarly, the production time for a 50 mg synthesis is also within the same time frame, averaging 10 days.
We use FedEx for both domestic and international shipments. For temperature-sensitive shipments, we offer either dry ice or blue ice (gel packs).
We are hiring
Are you ready to combine science with creativity? At Ella Biotech, you’ll join a close-knit team dedicated to advancing the life sciences. You’ll find a vibrant, respectful workplace where teamwork drives innovation and fun is always part of the mix.
Bring your talents to our team where every day is an exciting opportunity to make a difference in biotechnology and advance discovery. Let’s create the building blocks of tomorrow – together!
Contact us
„*“ zeigt erforderliche Felder an
