Modification Options
For ASO Gapmers
Antisense oligonucleotides (ASOs) are short, synthetic nucleic acid molecules designed to regulate gene expression by binding to complementary RNA sequences. However, unmodified ASOs are highly susceptible to nuclease degradation, which limits their stability and effectiveness. To enhance their therapeutic and research applications, chemical modifications are used to improve nuclease resistance, increase binding affinity, and reduce off-target effects or immune activation.
One specialized type of ASO is the gapmer, which consists of a central DNA region flanked by chemically modified RNA nucleotides. This unique design allows gapmers to form stable DNA-RNA hybrids with their target RNA sequences. Once bound, the hybrid is recognized by RNase H1, an enzyme that specifically degrades the RNA strand while leaving the DNA, or in this case the ASO, intact. As a result, gapmers effectively reduce the expression of target genes by promoting RNA degradation.
Advantages of Gapmers in gene silencing
Unlike siRNAs, which primarily act in the cytoplasm, gapmers can target both pre-mRNAs in the nucleus and mature mRNAs in the cytoplasm, giving them broader functionality in gene silencing.
Another key advantage of gapmers is their ability to enter cells via passive diffusion. Once inside, they remain stable in both the cytoplasm and nucleus, even at low concentrations. Modifications such as phosphorothioate (PTO) linkages enhance their stability by promoting strong interactions with serum proteins, reducing renal clearance and extending their circulation time. Additionally, gapmers exhibit high resistance to nuclease degradation when appropriately modified, ensuring prolonged activity in vivo. Unlike siRNAs, which require encapsulation for delivery, gapmers can often be administered without extensive protective formulations. Furthermore, recent advancements have explored the use of peptides to enhance gapmer transfection, while conjugation with small molecules such as GalNAc or fatty acids can also improve their targeted delivery to specific cells, potentially amplifying their therapeutic potential.
In contrast to unmodified single-stranded oligonucleotides, modern gapmers achieve high stability and nuclease resistance through precise chemical modifications such as phosphorothioate backbones, locked nucleic acids (LNA), and 2′-O-methoxyethyl (2′-MOE) sugar modifications. These enhancements not only protect gapmers from rapid degradation in biological fluids but also improve their binding affinity and specificity for target RNAs. While gapmers rely on RNase H1-mediated cleavage, which may be less efficient than the RNAi pathway used by siRNAs, optimizing the gap region and backbone chemistry can significantly boost their silencing potency and reduce off-target effects.
Let’s have a look of the main modification options that improve ASOs/ gapmer effectiveness and stability.
1. What are the key modifications aviailable, and how do they improve ASO stability?
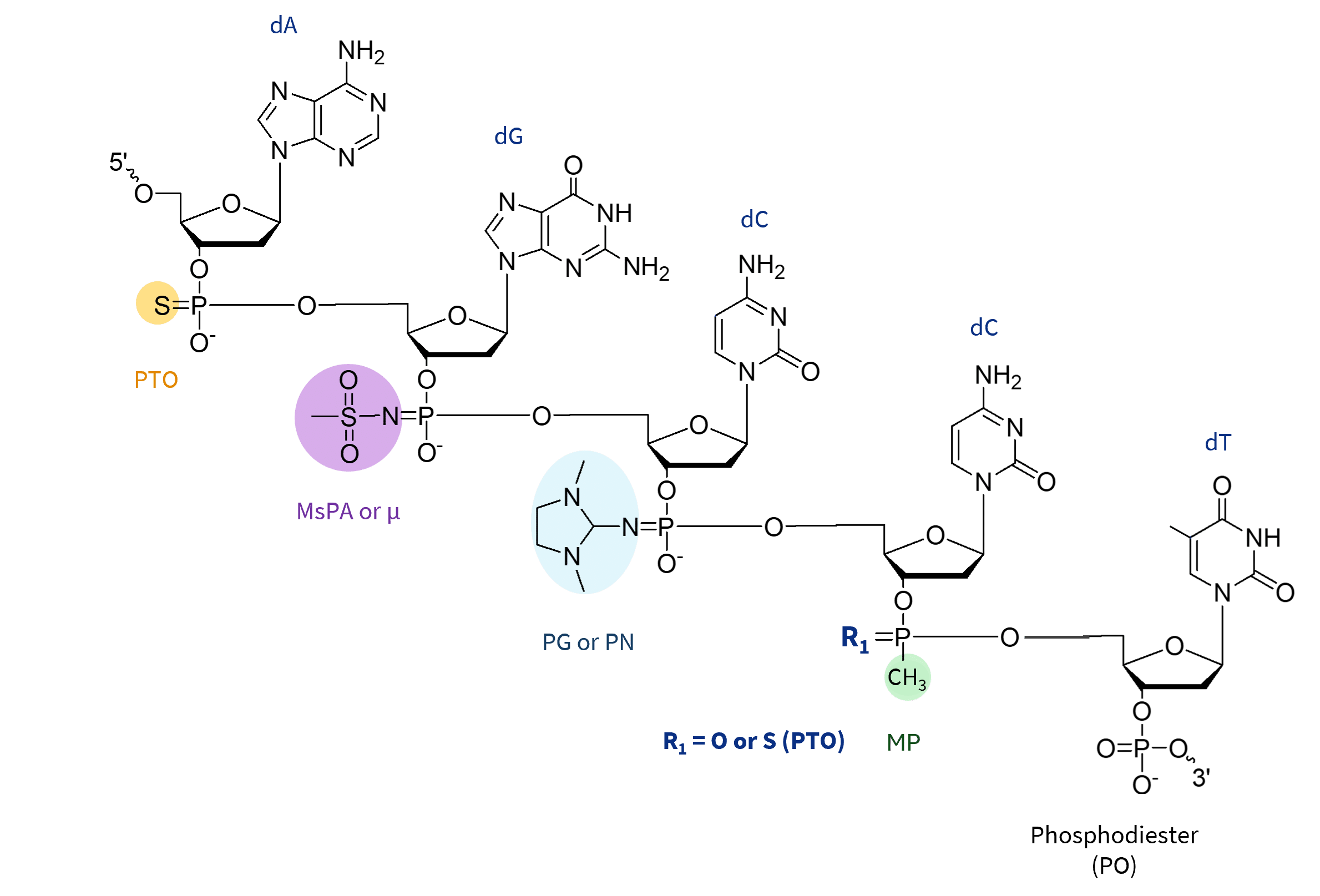
Backbone Modifications alter the phosphate linkage in ASOs, increasing their resistance to nucleases and improving stability in biological fluids. All of the displayed alterations to the internucleotide bonds result in the formation of stereoisomers. Here are the key types:
Phosphorothioate (PTO): This is the most widely used backbone modification, in which a non-bridging oxygen is replaced by sulfur. This creates a more robust molecular structure that resists nuclease degradation while maintaining RNase H1 activation. This modification improves in vivo half-life by increasing plasma protein binding, resulting in improved bioavailability. However, PTO linkages can increase non-specific protein interactions, potentially leading to toxicity or off-target effects. Nevertheless, PTO linkages are widely used in both the central DNA gap and flanking regions of gapmers, supporting efficient RNA cleavage in therapeutic applications.
Methylphosphonate (MP): Chemical modification of the phosphorus backbone in oligonucleotides, reducing the charge of the ASO and providing higher nuclease stability compared to PTO modifications. The modifications also reduce non-specific protein binding due to their neutral charge, potentially reducing hepatotoxicity. However, they also limit cellular uptake, reduce protein binding, and may not support RNase H1 activity, making them less effective for RNase H-dependent ASOs. While MP modifications can be useful in specific cases where increased stability and reduced off-target effects are required, their limitations have led to a preference for other chemical modifications, such as phosphorothioate (PTO) and 2′-MOE, in the development of ASOs and gapmers for therapeutic applications. Methylphosphonate modifications, unlike PTO, PG or MsPA, require unique methylphosphonamidite precursors, commercially limited to DNA nucleotides.
Mesyl Phosphoramidate (MsPA or µ ): The MsPA linkage is a modification in which the natural phosphodiester group is replaced with a methanesulfonyl (mesyl) group. It is designed to replace PTO bonds to further improve enzymatic stability. This modification helps to provide greater resistance to nucleases, reduce non-specific protein binding to minimize toxicity, and maintain RNA binding affinity and RNase H1 activity when strategically incorporated. MsPA is a promising alternative to PTO in ASOs, particularly in gapmer designs where they can be combined for improved stability with reduced inflammation.
Phosphoryl Guanidine (PG or PN): Phosphoryl guanidine (PN) linkages are a charge-neutral modification in which the phosphate group is replaced with a guanidine derivative, reducing the overall negative charge of the oligonucleotide. This improves cellular uptake, reduces off-target effects, and increases nuclease resistance while maintaining RNA binding affinity. In addition, they do not interfere with cellular uptake under carrier-free conditions when incorporated at specific positions, which is particularly advantageous for delivery to challenging tissues such as the central nervous system (CNS). The neutral charge introduced by PG modifications reduces electrostatic repulsion with cell membranes, which may contribute to improved cellular uptake and tissue distribution.
PG modifications are often used in conjunction with phosphorothioate (PTO) or phosphodiester (PO) linkages to optimize the stability, cellular uptake, and RNase H activity of therapeutic oligonucleotides.
Sugar Modifications
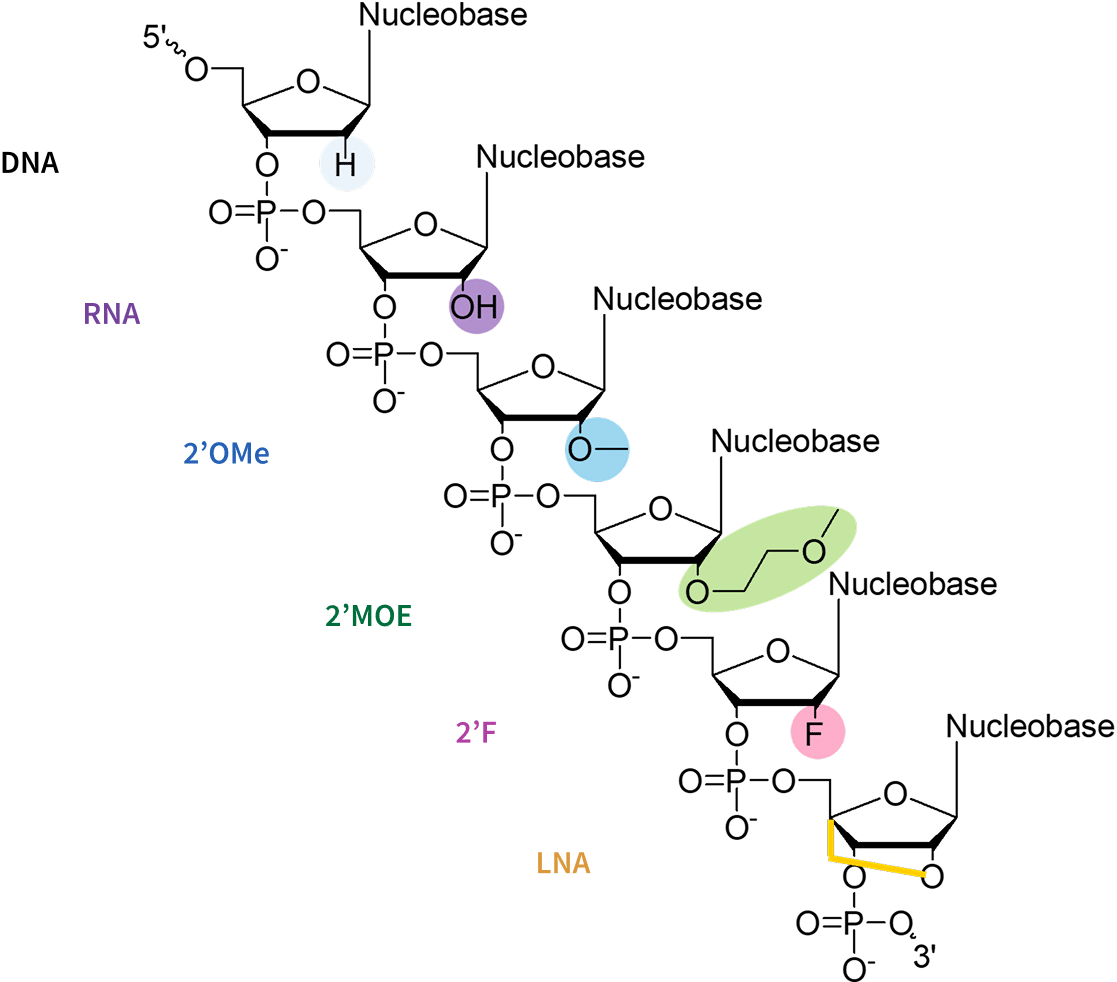
alter the ribose structure to improve binding affinity and stability. Key modifications include:
2′-O-Methyl (2′-OMe): The 2′-OMe modification replaces the 2′-hydroxyl group on the ribose with a methyl (-OCH3) group, increasing nuclease resistance, reducing immune stimulation, and moderately enhancing thermal stability and RNA binding affinity. It is a cost-effective, widely used in the flanking regions of gapmers to protect against degradation while maintaining RNase H1 compatibility for efficient target RNA cleavage. However, it provides only a moderate increase in thermal stability compared to 2′-Fluoro or LNA modifications.
2′-O-Methoxyethyl (2′-MOE): The 2′-O-methoxyethyl (2′-MOE) modification replaces the 2′-hydroxyl group of the ribose with a bulkier 2′-methoxyethyl (-OCH2CH2OCH3) group, providing greater steric hindrance and enhancing nuclease resistance than 2′-OMe. It increases RNA duplex stability, improves thermal stability (ΔTm ~0.9-1.7°C per modification) and pharmacokinetics, and reduces immune activation.
Gapmers with 2′-MOE modifications demonstrate more consistent gene knockdown and can help reduce hepatotoxicity in therapeutic applications. However, its bulkier structure may slightly reduce cellular uptake efficiency.
2′-Fluoro (2′-F): The 2′-Fluoro (2′-F) modification replaces the 2′-hydroxyl group of the ribose with a fluorine atom, which significantly enhances the RNA binding affinity due to the electronegativity of fluorine, which stabilizes base-pairing interactions. It also increases the resistance to nuclease by making the sugar-phosphate backbone more rigid.
In the gapmer design, 2′-F modifications in the flanking regions increase stability, improve potency and allow RNase H recruitment. It can also reduce immune stimulation and can be combined with other modifications such as 2′-O-Me or LNA to optimize efficacy and safety.
Locked Nucleic Acids (LNAs): Locked Nucleic Acids (LNAs) feature a methylene bridge connecting the 2′-oxygen and 4′-carbon atoms of the ribose, „locking“ the sugar in a rigid conformation. This structural rigidity dramatically increases thermal stability (ΔTm increase up to ~8°C per modification), RNA binding affinity, and nuclease resistance, making LNAs highly potent even at low doses. LNAs allow the use of shorter, highly specific oligonucleotides, proving valuable for challenging sequences.
LNAs are typically used in flanking regions to enhance hybridization. However, their strong binding affinity can increase toxicity, leading to off-target effects or immune responses. Extensive modifications beyond ~8 nucleotides can cause aggregation, requiring chimeric designs with other modifications.
Base Modifications
5-Methylcytosine (5Me-dC): This is a modification in which cytosine is methylated at the 5-position, offering several advantages for the design of ASOs and gapmers. The incorporation of 5Me-dC, particularly within CpG motifs, has been shown to reduce immunogenicity by minimizing Toll-like receptor 9 (TLR9)-mediated immune responses, which can be otherwise triggered by unmethylated CpG sequences. This modification also enhances ASO efficacy by slightly increasing duplex stability and thermal melting temperature, while maintaining compatibility with RNase H1 activity.
When combined with sugar modifications such as 2′-OMe, 2′-MOE, or LNA, 5Me-dC further stabilizes the ASO-RNA duplex and supports efficient, specific gene silencing with reduced risk of immune activation.
End Modifications
Exonucleases can degrade ASOs from the ends, reducing their effectiveness. End or capping modifications block this degradation:
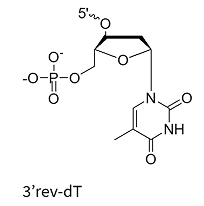
Inverted Bases (e.g., 3′-inverted dT): These modifications create a 3′-3′ linkage, effectively blocking 3′-exonuclease activity and preventing polymerase extension. This is a well-established strategy for protecting oligonucleotides from degradation. However, it should be noted that this method may slightly reduce hybridization efficiency at the modified end due to structural changes.
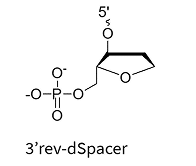
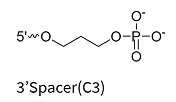
Chemical Spacers (e.g., rev-dSpacer, C3-Spacer): Hydrophilic spacers at the 3′ end inhibit exonuclease degradation and are commonly used in both research and therapeutic oligonucleotides. While these modifications are effective in improving nuclease resistance, they can sometimes slightly affect target binding affinity.
Other End Modifications (LNA, 2′-MOE, 2′-OMe, PTO, PG): Incorporating these modifications at the ends of gapmers and ASOs further increases resistance to exonuclease degradation.
2. How do I choose the best modification for my ASO design?
Depending on your specific application, choosing the right modification is important. If high nuclease resistance is required, LNAs or 2′-MOE modifications may be ideal. For activating RNase H, gapmers with PTO or MsPA backbones can be effective.
At Ella Biotech, our experts are here to assist you in selecting the modifications and combinations that might be the best fit for your particular goals and budget considerations while maintaining a smooth synthesis process.
Modification
Chemical Nature
Benefits
Drawbacks
Phosphorothioate (PTO or PS)
Sulfur replaces a non-bridging oxygen in the phosphate backbone
Enhances nuclease stability and protein binding; supports RNase H activity
Decreases Tm slightly; may cause non-specific protein binding and pro-inflammatory effects
Methylphosphonate (MP)
Non-ionic backbone modification
Increases nuclease resistance; reduces immune activation
Does not support RNase H activity, limiting its use in gapmer ASOs.
Mesyl-phosphoramidate (MsPA)
Phosphate modified with mesyl and amidate groups
Improves nuclease stability and reduces pro-inflammatory effects compared to PS modifications
May reduce antisense activity in some designs
Phosphoryl Guanidine (PN or PG)
Neutral backbone modification where a guanidine group replaces a phosphate oxygen atom
Enhances nuclease resistance and cellular uptake without lipid carriers; reduces overall charge, improving tissue penetration and pharmacokinetics
May reduce hybridization efficiency if overused
2′-OMe
Methyl group at the 2′-OH position
Cost-effective; enhances nuclease resistance; moderate Tm increase
Moderate RNase H inhibition
2′-MOE
Methoxyethyl group at the 2′-OH
Stronger nuclease resistance; lower toxicity; higher Tm increase
Bulkier
2′-F
Fluorine atom at the 2′-OH
Highest Tm increase; strong RNA-binding affinity
Higher toxicity risk with extensive use
LNA
Methylene bridge „locks“ ribose
Drastic Tm increase; exceptional nuclease resistance
Higher toxicity; aggregation issues
3′-Inverted dT
Reversed thymidine at the 3′ end
Blocks exonuclease degradation
May reduce hybridization efficiency
C3-Spacer
Hydrophilic spacer at the 3′ or 5′ end
Prevents exonuclease degradation; can attach functional groups like fluorophores or bioconjugates
May affect RNA-binding affinity
GalNAc conjugation
N-acetyl galactosamine conjugated to ASO
Enhances hepatocyte-specific delivery via ASGPR; improves potency 10-60 fold for liver targets
May slightly reduce activity if conjugation affects ASO structure
- Castanotto D, Sakurai K, Lingeman R, Li H, Shively L, Aagaard L, et al. 2015. Short antisense oligonucleotides and siRNAs: a common chemical scaffold for RNA interference and antisense inhibition. Nucleic Acids Res. 43(2):935–946.
- Lin Y, Qiu Q, Gill SC, Jayasena SD. 2015. Dual mechanisms of gene silencing by RNase H-dependent antisense oligonucleotides. Nucleic Acids Res. 43(2):932–944.
- Sørensen DR, Leirdal M, Sioud M. 2003. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 327(4):761–766.
- Stein CA, Castanotto D. 2017. FDA-approved oligonucleotide therapies in 2017. Mol Ther. 25(5):1069–1075.
- Kurreck J, Wyszko E, Gillen C, Rittner K, Erdmann VA. 2020. The NAA/LNA-gapmer approach: highly potent, stable, and specific antisense oligonucleotides for therapeutic applications. Nucleic Acids Res. 48(20):11679–11695.
- Lennox KA, Behlke MA. 2017. RNase H sequence preferences influence antisense oligonucleotide efficiency. Nucleic Acids Res. 45(13):7701–7716.
- Khvorova A, Watts JK. 2017. The chemical evolution of oligonucleotide therapies. Nat Biotechnol. 35(3):238–248.
- Seki M, Sato S, Matsumoto Y, Ohkubo S, Sato Y, et al. 2024. Stereodefined PMO-gapmers for improved safety and stability. bioRxiv. 2024 May 9:591947.
- Straarup EM, Fisker N, Hedtjärn M, Lindholm MW, Rosenbohm C, et al. 2012. α-L-LNA modified gapmer antisense oligonucleotides. Nucleic Acids Res. 40(2):e120.
- Smith C, Jones L, Patel R, Wang Y, Lee J, et al. 2024. Strategies to improve gapmer specificity. Mol Ther. 32(5):1001–1012.
- Yamamoto T, Nakatani M, Narukawa K, Harada-Shiba M. 2016. Ribonuclease H1-dependent hepatotoxicity of LNA gapmers. Sci Rep. 6:30377.
- Carlesso A, Hörberg J, Deganutti G, Reymer A, Matsson P. 2024. Structural dynamics of therapeutic nucleic acids with phosphorothioate backbone modifications. NAR Genomics Bioinform. 6(2):lqae058.
- Shen W, De Hoyos CL, Migawa MT, Vickers TA, Sun H, et al. 2020. Phosphorothioate modified oligonucleotide–protein interactions. Nucleic Acids Res. 48(10):5235–5253.
- De Koning MC, Filippov DV, van der Marel GA, van Boom JH. 1997. Synthesis and properties of methylphosphonate oligonucleotides. Nucleic Acids Res. 25(16):3310–3317.
- Prakash TP. 2011. An overview of sugar-modified oligonucleotides for antisense therapeutics. Chem Biodivers. 8(9):1616–1641.
- Vester B, Wengel J. 2004. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry. 43(42):13233–13241.
- Braasch DA, Corey DR. 2001. Locked nucleic acid (LNA): fine-tuning the recognition of DNA and RNA. Chem Biol. 8(1):1–7.
- RSC Med Chem. 2014. Antisense oligonucleotides: modifications and clinical trials. 5(9):1029–1045.
- Obika S, Nanbu D, Hari Y, Morio K, In Y, Ishida T, Imanishi T. 1997. Synthesis of 2′-O,4′-C-methyleneuridine and -cytidine. Novel bicyclic nucleosides having a fixed C3′-endo sugar puckering. Tetrahedron Lett. 38(50):8735–8738.
- Koshkin AA, Singh SK, Nielsen P, Rajwanshi VK, Kumar R, Meldgaard M, Olsen CE, Wengel J. 1998. LNA (locked nucleic acid): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerization, and unprecedented nucleic acid recognition. Tetrahedron. 54(14):3607–3630.
- Crooke ST, Witztum JL, Bennett CF, Baker BF. 2019. RNA-targeted therapeutics. Cell Metab. 29(2): 287–300.
- Lennox KA, Behlke MA. 2020. Chemical modification and design of antisense oligonucleotides for therapeutic applications. Annu Rev Pharmacol Toxicol. 60:185–203.
We are hiring
Are you ready to combine science with creativity? At Ella Biotech, you’ll join a close-knit team dedicated to advancing the life sciences. You’ll find a vibrant, respectful workplace where teamwork drives innovation and fun is always part of the mix.
Bring your talents to our team where every day is an exciting opportunity to make a difference in biotechnology and advance discovery. Let’s create the building blocks of tomorrow – together!
Contact us
„*“ zeigt erforderliche Felder an
