Modification
Options For siRNA
Small interfering RNAs (siRNAs) are short, double-stranded RNA molecules. They are usually 20–25 nucleotides long. siRNAs mediate post-transcriptional gene silencing. They work by being incorporated into the RNA-induced silencing complex (RISC), which facilitates the recognition and degradation of complementary messenger RNA (mRNA) targets. This binding leads to the cleavage and subsequent degradation of the target mRNA, effectively silencing the gene.
Advantages of siRNA
One of the major advantages of siRNA is its high specificity, as sequence complementarity ensures precise targeting of mRNA transcripts. This specificity reduces off-target effects at low concentrations (1 nM) , making siRNA an efficient tool for gene silencing. Unlike other gene silencing approaches, siRNAs act directly in the cytoplasm where proteins are made, ensuring a rapid and direct effect.
To increase their stability and therapeutic efficacy, siRNAs often undergo chemical modifications, such as phosphorothioate (PTO) linkages and 2’-O-methyl (2′-OMe) substitutions. These modifications enhance resistance to enzymatic degradation, improve pharmacokinetic properties, and reduce immunogenicity. In addition, siRNAs are generally more stable than single-stranded antisense oligonucleotides (ASOs) when present in blood or inside cells, making them a good choice for gene silencing therapies.
Limitations of siRNA
Despite their advantages, siRNAs face significant challenges, particularly in terms of cellular delivery. Due to their double-stranded nature, large size, and negative charge, siRNAs have poor membrane permeability, requiring specialized delivery systems for efficient cellular uptake. Lipid nanoparticles (LNPs) and conjugates such as N-acetylgalactosamine (GalNAc) have been developed to facilitate targeted delivery, protect siRNAs from degradation, and enhance their intracellular uptake.
Another concern associated with siRNAs is the potential for off-target effects. These effects can occur when siRNAs bind to partially complementary sequences in unintended mRNA, particularly at high siRNA concentrations of >10nM. Additionally, siRNAs can trigger immune responses, particularly if recognized by innate immune sensors such as Toll-like receptors (TLRs). Furthermore, naked siRNAs are prone to rapid degradation by nucleases in biological fluids, limiting their half-life and therapeutic potential.
Due to these challenges, effective siRNA-based therapies require strategic chemical modifications to enhance nuclease resistance, improve binding affinity, and minimize immune activation. Additionally, advanced delivery systems, such as lipid nanoparticles and GalNAc conjugates, are essential to protect siRNAs and ensure efficient intracellular delivery, maximizing their therapeutic potential.
Below are the main modification options that commonly used to improve the effectiveness and stability of siRNAs.
1. What are the key modifications that enhance siRNA stability?
Several types of modifications can improve siRNA performance, including:
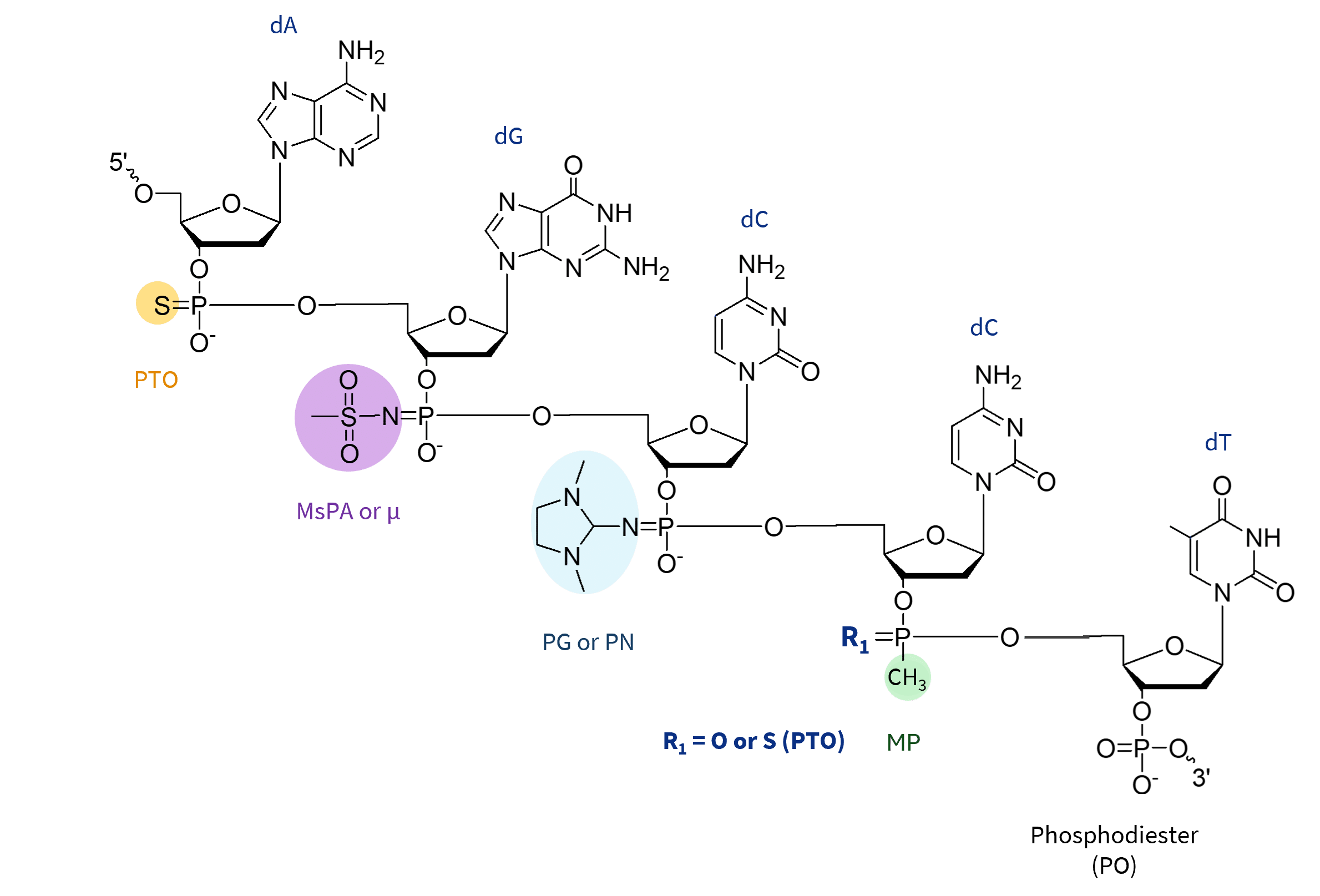
Backbone Modifications
Please note that both PTO as well as MP bonds result in the formation of stereoisomers.
Phosphorothioate (PTO): The phosphorothioate (PTO) modification replaces a non-bridging oxygen atom in the phosphate backbone with a sulfur atom, increasing resistance to exonucleases and endonucleases while stabilizing the backbone. This modification improves cellular uptake by reducing electrostatic repulsion with cell membranes due to its slightly negative charge. PTO modifications also enhance the interaction of oligonucleotides with serum proteins, resulting in reduced renal clearance and prolonged circulation time.
While this increases cellular uptake and overall stability of the oligonucleotide, it can also lead to potential off-target effects due to unintended interactions with cellular components. In addition, extensive PTO modification can induce cytotoxic effects and reduce gene silencing efficiency. To mitigate these risks, a balanced approach – such as partial PTO modification, particularly in the 3′-terminal region – combined with other chemical modifications can help optimize performance while minimizing off-target effects.
Methylphosphonate (MP): MP modifications replace a non-bridging oxygen atom with a methyl group, effectively removing the negative charge from the phosphate backbone. The primary advantages of this modification include increased nuclease stability, reduced non-specific protein binding, and a potential reduction in immunogenicity. However, MP modifications also have significant limitations, such as reduced cellular uptake due to the lack of charge, which can affect the overall efficacy of siRNA. In addition, MP modifications do not efficiently support RNase H activity or RISC incorporation, which are essential for siRNA function. Methylphosphonate modifications, unlike PTO, PG or MsPA, require unique methylphosphonamidite precursors, commercially limited to DNA nucleotides.
While MP can be useful as part of a broader strategy to optimize siRNA therapeutics, often in combination with other chemical modifications, it is generally less common in clinical siRNA development compared to other modifications such as phosphorothioate (PTO), 2′-O-methyl, and 2′-fluoro.
5′-(E)-Vinylphosphonate (5′-E-VP): 5′-(E)-Vinylphosphonate (5′-E-VP) involves the introduction of a vinylphosphonate group at the 5′ end of the siRNA guide strand. This mimics the native 5′-phosphate required for RISC loading, and the modification boosts recognition by Argonaute 2 (AGO2). It also protects the 5′ end from exonuclease degradation and increases metabolic stability.
Although this modification has been shown to enhance RISC incorporation efficiency and RNAi activity while reducing off-target cleavage.
Please inquire for 5′-E-VP modification as it is not in our standard offering.
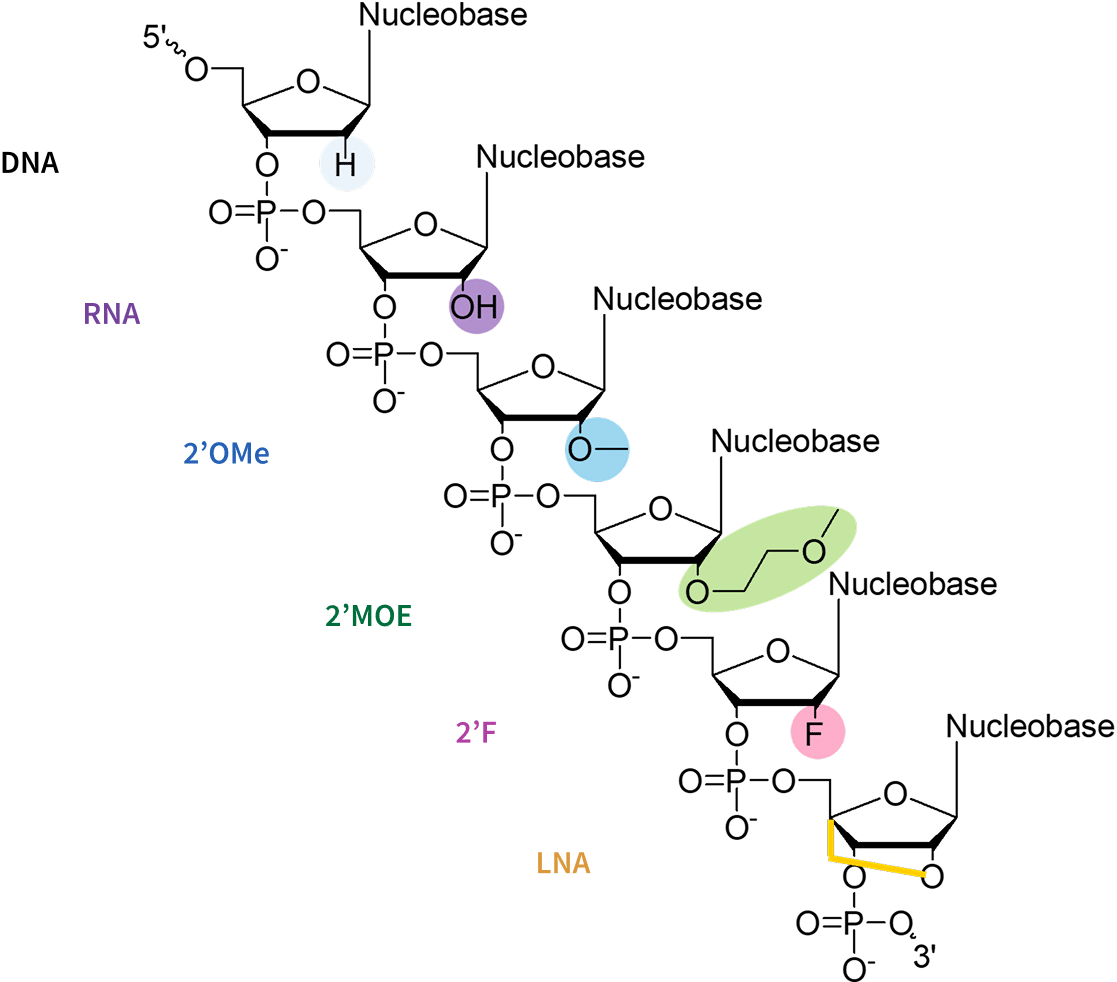
Sugar Modifications
Sugar modifications alter the ribose structure to improve binding affinity and stability. Key modifications include:
2′-O-Methyl (2′-OMe): The 2′-O-methyl (2′-OMe) modification involves the introduction of a bulky methyl group at the 2′ position of the ribose, resulting in a significant enhancement of nuclease resistance, and a reduction in immune activation by decreasing Toll-like receptor (TLR) recognition. By replacing the 2′-OH group, this modification also prevents the ribose from participating in nucleophilic reactions, which stabilizes the siRNA duplex without compromising its functionality. While it occurs naturally in RNA and is relatively non-toxic, extensive incorporation may slightly reduce RNAi efficacy. Combination with other modifications, such as phosphorothioate (PTO) backbones can further enhance stability and reduce immune activation.
2′-O-Methoxyethyl (2′-MOE): The 2′-Methoxyethyl (2′-MOE) modification adds a methoxyethyl group to the ribose, further enhancing nuclease resistance, binding affinity and siRNA half-life in biological systems. It also increases serum stability by approximately 30-40% while reducing immune recognition and off-target effects. This modification provides stronger nuclease resistance than 2′-OMe and increases Tm by ~0.9-1.7°C per modification. However, its bulkier structure may affect RISC loading efficiency and it is more expensive to synthesize than simpler modifications such as 2′-OMe.
2′-Fluoro (2′-F): 2′-Fluoro (2′-F) modification replaces the hydroxyl group of the ribose with fluorine, which greatly enhances RNA duplex stability by increasing base stacking interactions and hydrogen bonding with the target mRNA, significantly increases melting temperature (Tm) by ~2.5°C per modification, strengthens siRNA binding to Argonaute 2 (AGO2) in the RISC complex, and improves metabolic stability. However, due to its high binding affinity, excessive use may increase toxicity and lead to off-target effects.
Locked Nucleic Acids (LNAs): Locked Nucleic Acid (LNA) modification introduces a methylene bridge between the 2′-O and 4′-C positions of the ribose, locking the sugar in a rigid conformation that dramatically increases thermal stability and nuclease resistance. It significantly increases RNAi activity (5-10 fold), improves mismatch discrimination, and provides the highest Tm boost (up to ~8°C per modification). However, its strong binding affinity increases the risk of toxicity, and full modification beyond ~8 nucleotides can cause oligonucleotide aggregation, requiring careful design in siRNA applications.
End Modifications
End modifications are important to optimize siRNA stability, reduce immune activation, and maintain effective gene silencing and can be accomplished by 5′-capping and/or 3′-capping.
5′-capping modifications are achieved by adding chemically modified groups to the 5′-end of siRNAs. These caps serve several purposes: they protect the siRNA from exonuclease degradation, thus increasing the stability of the siRNA, and they prolong its activity by extending its half-life in biological systems. The modified caps, such as inverted thymidine, which protects against exonuclease activity and maintains structural integrity, also help to reduce recognition by the host’s pattern recognition receptors (PRRs), hence minimizing immune responses.
However, the selection of 5′ modifications in siRNA design must carefully balance stability and compatibility with RISC loading, as improper modifications can interfere with RISC recognition and reduce silencing efficiency.
3′-Capping modifications in siRNAs are designed to enhance stability and functionality by protecting the 3′ end from degradation by exonucleases, which are highly active in the cellular environment. These modifications extend the half-life of the siRNA, ensuring prolonged gene silencing activity, while maintaining the structural integrity of the duplex, which is critical for efficient RISC loading and target recognition.
For example, inverted deoxythymidine (idT) is often used at the 3′ end to block enzymatic degradation while maintaining compatibility with RNA-induced silencing complex (RISC) loading.
Beyond simple capping, several advanced end modification strategies are used to optimize siRNA performance.
Phosphorothioate (P-S) linkages : Commonly used at both ends, increase nuclease resistance, although excessive modifications can lead to cytotoxicity.
Fluorescent labels or bioconjugates: Often incorporated at the 3′ end, facilitate siRNA tracking in cellular assays without interfering with RISC loading.
Terminal overhangs: They are typically siRNAs with 2 nucleotide (2 nt) 3′ overhangs such as dTdT and UU on one or both strands, enhance compatibility with RISC, and chemical modifications at these sites can further improve stability without compromising activity.
Modification
Chemical Nature
Benefits
Drawbacks
Phosphorothioate (PTO or PS)
Sulfur replaces a non-bridging oxygen in the phosphate backbone
Increased nuclease resistance, better cellular uptake, prolonged circulation
Potential off-target effects, reduced gene silencing with extensive modification
Methylphosphonate (MP)
Methyl group replaces a non-bridging oxygen in the phosphate backbone
Increased nuclease stability, reduced non-specific binding and immunogenicity
Reduced cellular uptake, inefficient support of RNase H activity or RISC incorporation.
5′-E-VP
Vinylphosphonate group at the 5′ end of the siRNA guide strand
Boosts AGO2 recognition, protects 5′ end from degradation
Protection limited to 5′ end
2′-OMe
Methyl group at the 2′-OH position
Enhances nuclease resistance, stabilizes the siRNA duplex and reduces immune activation
Extensive incorporation may slightly reduce RNAi efficacy.
2′-MOE
Methoxyethyl group at the 2′-OH
Stronger nuclease resistance, reduces immune recognition
Bulkier structure may affect RISC loading, more expensive than 2′-OMe
2′-F
Fluorine atom at the 2′-OH
Increases RNA duplex stability, strengthens AGO2 binding and improves metabolic stabillity
Excessive use may increase toxicity and increase off-target effects.
LNAs
Methylene bridge „locks“ ribose
Provides the highest Tm boost, increases RNAi activity, and mismatch discrimination
Increased risk of toxicity and aggregation with > 8nt modification
End Modification
5′-Capping
Protects from degradation, increases stability, minimizes immune responses
Must carefully balance stability and compatibility with RISC loading.
End Modification
3′-Capping
Extends half-life, maintains duplex integrity
Advanced End Modifications
Phosphorothioate (P-S) linkages, Fluorescent labels or bioconjugates, Terminal overhangs (dTdT and UU)
Increased nuclease resistance, facilitates siRNA tracking, enhances compatibility with RISC.
Excessive P-S modifications can lead to cytotoxicity, modifications must not interfere with RISC loading
GalNAc Conjugation
N-acetylgalactosamine covalently linked to the siRNA
Targets liver cells, improves silencing in hepatocytes
Limited to hepatocytes, may affect uptake and RISC loading
2. Can I combine different type of modifications in siRNA design?
Sure. When designing siRNAs, you can strategically combine the placement of sugar and backbone modifications to enhance siRNA function while maintaining RISC processing efficiency. Below are just two examples.
Alternating Sugar Modifications (2′-F and 2′-OMe): The use of alternating 2′-F and 2′-OMe modifications can improve structural integrity, optimize strand asymmetry for efficient guide strand selection, and reduce off-target effects. Depending on the specific siRNA sequence and desired properties, the exact placement of these modifications may vary.
For example, the modification of pyrimidines (U/C) with 2′-F and purines (A/G) with 2’-OMe in the guide strand is one of the possible configurations. This configuration is used in FDA-approved drugs like Patisiran (Onpattro®) and Lumasiran (Oxlumo®) to balance stability and activity.
Backbone + Sugar Modifications (PTO + 2′-OMe regions): To increase overall siRNA stability while maintaining AGO2 cleavage activity and minimizing non-specific immune activation, PTO modifications increase nuclease resistance, while 2′-OMe modifications can reduce immunogenicity.
For example, Givosiran (Givlaari®) uses a mix of 2′-OMe, 2′-F, and PTO to achieve prolonged circulation and liver-targeted delivery.
However, it’s important to note that extensive PTO modification (>50% of backbone) in siRNAs can potentially interfere with RISC loading and increase cytotoxicity, so their use must be carefully balanced.
3. How do I choose the best modification for my ASO design?
Depending on your specific application, choosing the right modification is important. If high nuclease resistance is required, LNAs or 2′-MOE modifications may be ideal. For activating RNase H, gapmers with PTO or MsPA backbones can be effective.
At Ella Biotech, our experts are here to assist you in selecting the modifications and combinations that might be the best fit for your particular goals and budget considerations while maintaining a smooth synthesis process.
- Seitzer N, An D, Romin Y, Boyko V, Soll RM. 2023. GalNAc-lipid nanoparticles enable non-LDLR-dependent hepatic delivery of a CRISPR base editor. Nat Commun. 14(1):37465.
- Sasaki S, Guo S, Naito M, et al. 2020. 2′-OMe-phosphorodithioate-modified siRNAs show increased stability and enhanced potency in vivo. Nucleic Acids Res. 48(15):8251–8261.
- Tabernero J, Shapiro GI, Lorusso PM, et al. 2012. First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov. 3(4):406–417.
- Khvorova A, Watts JK. 2017. The chemical evolution of oligonucleotide therapies. Nat Biotechnol. 35(3):238–248.
- Kurreck J, Wyszko E, Gillen C, Rittner K, Erdmann VA. 2020. The NAA/LNA-gapmer approach: highly potent, stable, and specific antisense oligonucleotides for therapeutic applications. Nucleic Acids Res. 48(20):11679–11695.
- De Koning MC, Filippov DV, van der Marel GA, van Boom JH. 1997. Synthesis and properties of methylphosphonate oligonucleotides. Nucleic Acids Res. 25(16):3310–3317.
- Schirle NT, Kinberger GA, Murray HM, et al. 2013. siRNA carrying an (E)-vinylphosphonate moiety at the 5′ end of the guide strand augments gene silencing by enhanced binding to human Argonaute-2. Nucleic Acids Res. 45(6):3528–3536.
- Chakraborty C, Sharma AR, Sharma G, Doss CGP, Lee S-S. 2017. Therapeutic advances of chemically modified siRNA: challenges and opportunities. Front Genet. 8:137.
- Bramsen JB, Laursen MB, Damgaard CK, et al. 2006. Improved silencing properties using small internally segmented interfering RNAs. Nucleic Acids Res. 34(21):5886–5897.
- Liu J, Guo S, Cinier M, et al. 2017. Methoxyethyl group improves the specificity and activity of siRNAs. Nucleic Acids Res. 45(19):11254–11267.
- Chen Y, Shen X, Asanuma H. 2015. Chemical modifications of siRNA for in vivo delivery and therapeutic development. Med Res Rev. 35(2):136–162.
- Vester B, Wengel J. 2004. LNA (locked nucleic acid): high-affinity targeting of complementary RNA and DNA. Biochemistry. 43(42):13233–13241.
- Bramsen JB, Laursen MB, Nielsen AF, Hansen TB, Bus C, Langkjaer N, Babu BR, Højland T, Abramov M, Van Aerschot A, et al. 2009. A large-scale chemical modification screen identifies design rules to generate siRNAs with high activity, high stability and low toxicity. Nucleic Acids Res. 37(9):2867–2881.
- Chen Y, Shen X, Asanuma H. 2015. Chemical modifications of siRNA for in vivo delivery and therapeutic development. Med Res Rev. 35(2):136–162.
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 411(6836):494–498.
- Advances in structural-guided modifications of siRNA. 2024. Sci Direct.
- Therapeutic siRNA: state of the art. 2020. Nature.
- Naito M, Ui-Tei K, Saigo K, et al. 2014. 2′-OMe-phosphorodithioate-modified siRNAs show increased stability and enhanced potency. Nat Commun. 5:5522.
- Advances in siRNA therapeutics and synergistic effect on siRNA activity using chemical modifications. 2022. PMC.
We are hiring
Are you ready to combine science with creativity? At Ella Biotech, you’ll join a close-knit team dedicated to advancing the life sciences. You’ll find a vibrant, respectful workplace where teamwork drives innovation and fun is always part of the mix.
Bring your talents to our team where every day is an exciting opportunity to make a difference in biotechnology and advance discovery. Let’s create the building blocks of tomorrow – together!
Contact us
„*“ zeigt erforderliche Felder an
