Designing Oligo With Multiple Modifications
Key Considerations For A Logical Synthesis Pathway
What do I need to consider when I design my oligonucleotides to incorporate multiple modifications?
During oligonucleotide synthesis, modifications can be introduced at various stages to improve the functionality, stability, or specificity of the oligo. Each stage offers different opportunities for modification, but also presents unique challenges. When designing oligos with multiple modifications, it’s important to carefully consider the compatibility of the modifications with each phase, their impact on synthesis efficiency, and their effect on the functionality of the oligo. Balancing these factors is critical to producing high quality, functional oligos suitable for specific applications.
At Ella Biotech, we offer comprehensive support throughout the entire process of designing and synthesizing modified oligonucleotides. Our expert team provides guidance on modification selection, compatibility assessment, and optimization strategies, ensuring the production of high-quality, reproducible oligos tailored to your specific research needs. This service is included as part of our commitment to delivering exceptional value and scientific excellence to our customers.
Below are the four main phases where modifications can be incorporated into oligonucleotides:
The Four Phases of Modification Opportunities in Oligonucleotide Synthesis
1. Pre-Synthesis Modifications
Pre-synthesis modifications occur before the first nucleotide is even added to the oligonucleotide chain. In this stage, solid supports such as CPG (controlled pore glass) or polystyrene are functionalized with specific chemical groups. These modifications become an integral part of the synthetic platform. Typical examples for this are quencher molecules or 3’ phosphorylation.
2. During Synthesis
Modification during synthesis typically is the most straightforward and efficient. This phase involves directly incorporating phosphoramidite building blocks that carry the desired modifications into the synthetic cycle. For example, phosphoramidites with fluorescent tags or specific chemical groups (such as biotin) can be easily added to the oligonucleotide chain during synthesis. It is also during synthesis that the phosphodiester bond is modified, e.g. phosphorothioate bonds are sulfurized.
At Ella Biotech, this method is favored for its simplicity, high efficiency, and reliability.
3. Post-Synthetic Solid Phase Modifications
After the oligonucleotide is synthesized but still attached to the solid support, post-synthetic solid-phase modifications can be carried out.
This method uses orthogonal protection strategies, such as 9-fluorenylmethoxycarbonyl (Fmoc) and trifluoroacetic acid (TFA), to selectively modify functional groups without affecting others. By leaving certain groups in place, modifications can be introduced in a controlled manner before complete deprotection is achieved. While most protecting groups are removed during cleavage from the solid support, some orthogonal strategies allow selective deprotection even after the oligonucleotide is in solution. The choice of protective groups and modification techniques depends on the specific chemical properties and functional requirements of the final oligonucleotide.
4. Post-Synthetic Liquid Phase Modifications
Modification can also be carried out once the synthesized oligo has been cleaved from the solid support and is free in solution.
At this stage, click chemistry reactions, nucleophilic labeling (NHS ester chemistry) and site-specific conjugations can be used to introduce various modifications to the oligo. The liquid phase provides an optimal environment for performing more complex or delicate reactions and is especially valuable for adding modifications that might otherwise be damaged during the oligonucleotide synthesis process.
Key Challenges in Choosing The Suitable Modifications
While there are many opportunities for modification at each stage, several challenges must be carefully considered when designing a modified oligonucleotide:
1. Availability of Modifications
One significant challenge is the availability of the building blocks required for specific modifications. The economic and synthetic challenges of producing modified building blocks often limit their availability. Modifications that don’t have a lot of commercial demand may not be viable for suppliers, while complex synthesis processes can be costly and time-consuming. Additionally, if there are no established synthetic routes, producing the necessary reagents becomes difficult. These factors create an „economic filter,“ meaning that only modifications with strong demand and simpler synthesis paths become widely available.
2. Stability of Modifications
Another critical consideration in modification design is stability. Modifications must be able to survive the harsh conditions of oligonucleotide synthesis, particularly during deprotection steps and the cleavage from solid support. This is particularly important for longer oligos, which often require extended deprotection times that can potentially damage those more sensitive modifications. Therefore, any modification introduced must be stable under these rigorous conditions. Testing for stability is crucial to ensure that the desired modification does not degrade or lose functionality during the synthesis process.
3. Orthogonality of Modifications
Orthogonality refers to the concept of non-interference between different functional groups or reactive sites in a molecule during chemical reactions. It is achieved by chemo-, regio- and stereocontol in reactions which is exceedingly hard when attempted on large and complex molecules such as oligonucleotides. Achieving orthogonality is essential for oligos with multiple modifications, as it ensures that the reactions can occur independently without unwanted cross-reactions.
Orthogonality can be achieved in three main ways:
- Temporal Separation: Modifications are introduced at different stages of synthesis, preventing simultaneous reactions from interfering with each other. This is achieved by selective removal of specific protection groups.
- Chemical Selectivity: Using reaction conditions or reagents that are specific to one modification but not the other, ensuring that each modification is introduced without affecting the others.
For example, copper-catalyzed azide alkyne cycloaddition (CuAAC) is a widely used method to achieve orthogonal modifications. By carefully selecting specific chemical partners and controlling reaction conditions, multiple modifications can be introduced into the oligonucleotide with high precision. Alternative click chemistry techniques, such as SPAAC (strain-promoted alkyne-azide cycloaddition), iEDDA (inverse electron-demand Diels-Alder), and others, offer additional tools for achieving orthogonality.
Three Major Chemistries Used For Introducing Modifications:
There are three primary chemistries used for introducing modifications: phosphoramidites, nucleophilic labeling, and click chemistry. Each method has its advantages and limitations, which influence their application in different stages of oligonucleotide synthesis.
1. Phosphoramidite Chemistry: The Standard for Oligo Synthesis
Phosphoramidite chemistry is the gold standard method for oligonucleotide synthesis, widely recognized for its efficiency, versatility, and ability to incorporate a range of modifications directly during the synthesis process. This approach is used extensively for creating both standard and modified oligonucleotides, making it the foundation of oligonucleotide modification.
How Phosphoramidite Chemistry Works
The phosphoramidite synthesis cycle consists of four main steps: deblocking, coupling, capping, and oxidation. These steps allow for the systematic addition of nucleotides to the growing oligonucleotide chain. In this method, phosphoramidite building blocks—which are chemically modified nucleotides—are introduced to the growing chain one at a time, allowing for precise control over the sequence.
Modifications can be incorporated at various positions within the oligonucleotide, including:
- 5’-end modifications (e.g., fluorescent tags or functional groups)
- Internal modifications (e.g., base substitutions or sugar modifications)
- 3’-end modifications (e.g., quencher)
This ability to add modifications directly during the synthesis process is one of the reasons why phosphoramidite chemistry is considered the most convenient and efficient method for oligo synthesis. At Ella Biotech, this approach is preferred because of its simplicity and effectiveness.
Orthogonality of Phosphoramidite Building Blocks
Phosphoramidite chemistry offers an important advantage: the building blocks used in this process are generally orthogonal to each other, allowing various modifications to be incorporated into oligonucleotides without interference. Due to the well-established protection group chemistry most modifications introduced with phosphoramidites have similar deprotection and cleavage conditions and therefore are compatible to each other.
phosphoramidite
DMT protection group
Cy5
Example of a phosphoramidite: Cy5 Phosphoramidite
Challenges with Deprotection and Cleavage Conditions
While Phosphoramidite chemistry offers numerous advantages; however, deprotection and cleavage conditions present a limitation for certain modifications. These steps involve treating oligos in harsh conditions, such as strong acids or bases. Some modifications simply cannot survive these conditions.
For instance, fluorescent dyes with absorption above 640 nm (such as Cy5) are typically too sensitive to these conditions when incorporated directly as phosphoramidites. These dyes may degrade under the deprotection conditions used in standard oligo synthesis. As a result, post-synthetic modification techniques are required.
Limitations with Longer Oligonucleotides
As the oligonucleotide length increases, the deprotection and cleavage conditions become more aggressive. This can further limit the types of modifications that can be successfully incorporated. For example, Cy5 phosphoramidite can only be used in oligonucleotides up to approximately 45 bases long. Beyond this length, the harsher conditions required for deprotection and cleavage can destroy sensitive modifications such as dyes.
For longer sequences, it is often necessary to rely on post-synthetic modification methods, such as click chemistry or nucleophilic labeling, to introduce modifications that could not withstand the harsh conditions of synthesis.
2. Nucleophilic Labeling: The Second Line of Attack
The second most popular modification methods is nucleophilic labeling, with maleimide chemistry offering an additional powerful tool alongside NHS (N-hydroxysuccinimide) and TFP (tetrafluorophenyl) ester techniques. This approach allows for the attachment of various labels, including fluorescent tags and other functional molecules, to oligonucleotides.molecules, to oligonucleotides.
How Nucleophilic Labeling Works
For this process to work, the oligo must have a free reactive group, typically an amino or thiol group, which is incorporated into the oligonucleotide sequence during solid phase synthesis using phosphoramidite chemistry. An amino group can be attached to regular nucleobases (such as adenine, thymine, cytosine, or guanine) or even to non-nucleosidic molecules, providing flexibility in label positioning. Thiol groups are introduced via synthetic linkers or by modification of existing nucleotides.
The labeling process involves the reaction between the nucleophilic reactive group on the oligonucleotide and electrophilic labeling reagents, which include NHS esters, TFP esters, and maleimides (which are specific for thiol groups). This reaction results in the formation of a covalent bond between the oligonucleotide and the desired label.
Orthogonality And The Limitations
However, nucleophilic labeling has one limitation: it is not orthogonal to itself. This means that if multiple amino groups are introduced into a single oligonucleotide, it is difficult to selectively label just one of them without affecting the others. This problem arises because nucleophilic labeling uses the same functional group (the amino group) for all labeling reactions, making it difficult to control where labeling occurs. To overcome this, one approach is to use selective deprotection. After the oligonucleotide is synthesized, one amino group can be selectively deprotected (while the others remain protected) to allow the labeling reaction to occur at just that site. This step, known as solid-phase labeling, typically involves attaching the label to the oligonucleotide after it has been synthesized but before it is cleaved from the solid support.
However, solid-phase labeling has several drawbacks. First, it often has low labeling efficiency, meaning that fewer oligonucleotides will successfully incorporate the label. In addition, the process requires considerable manual effort to carefully control which amino group is deprotected and to ensure that other groups remain protected. Another challenge is that the modification being added must be stable under the conditions required for deprotection, which adds complexity to the process. Despite these drawbacks, solid-phase labeling remains a valuable technique in certain applications where site-specific labeling is crucial.
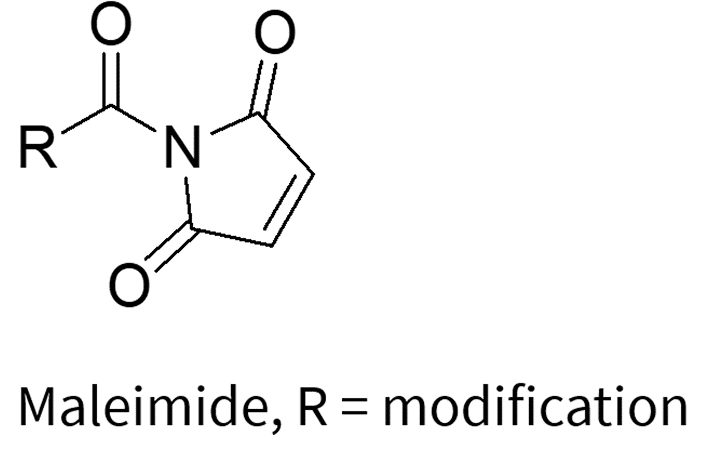
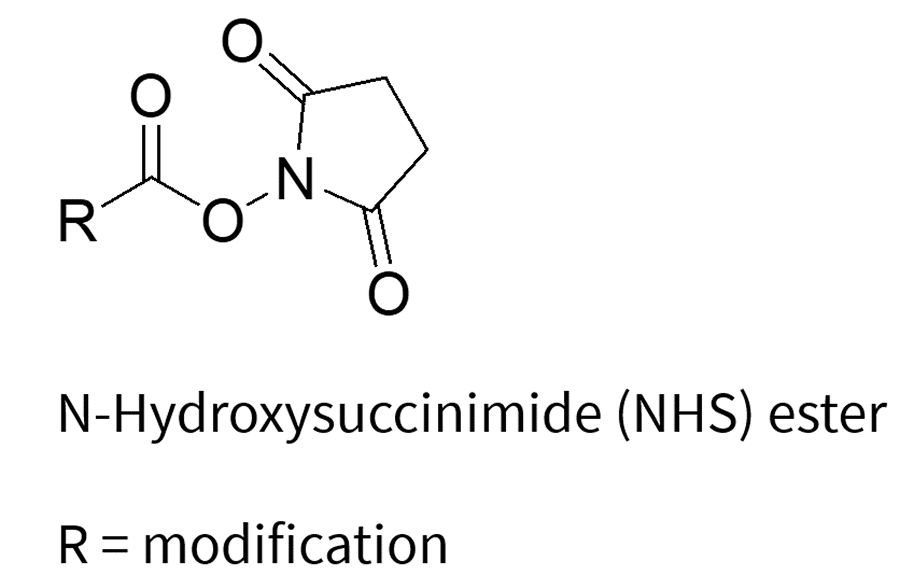
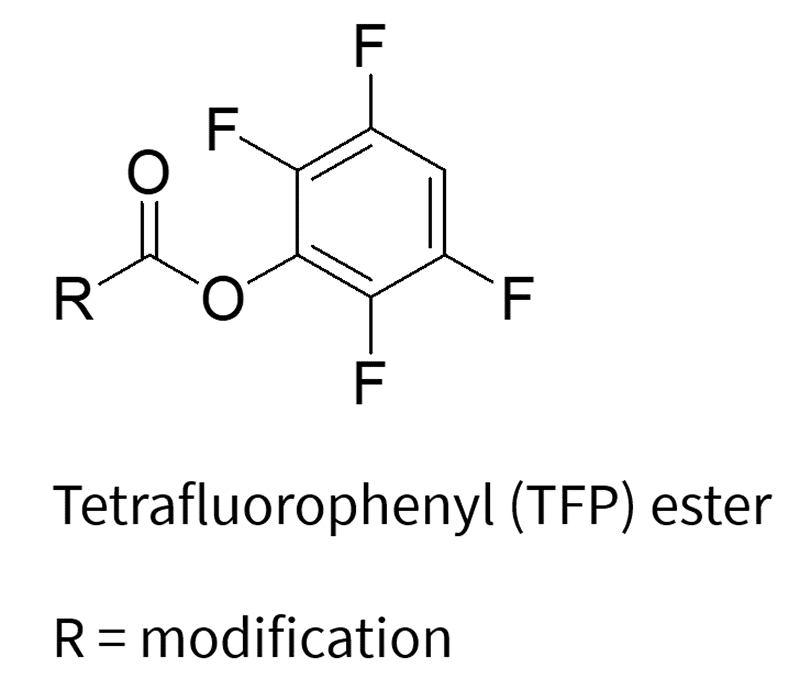
3. Click Chemistry: The Modern Alternative
The synthesis of highly modified oligonucleotides often requires a combination of techniques to introduce the desired modifications. One common strategy is the use of click chemistry, which can be applied either during synthesis or post-synthesis.
Click Chemistry Overview
Click chemistry involves a series of chemical reactions that enable the conjugation of two molecules in a highly efficient and selective manner. For oligonucleotides, modifications can either be incorporated during the synthesis process (using building blocks such as CuAAC alkynes or 5’TCO) or added post-synthesis using compounds such as DBCOs, azides, methyltetrazines, and TCOs. These modifications are then reacted with their corresponding counterparts to form the final conjugate.
For more information on this topic, please refer to the page Click Chemistry.
Challenges in Synthesis of Highly Modified Oligonucleotides
The synthesis of highly modified oligonucleotides requires a deep understanding of the underlying chemistry and a broad knowledge of available building blocks, as well as the chemistry of cleavage and protection groups.
Let’s consider a hypothetical example where we want to synthesize a modified oligonucleotide with multiple functional groups.
Example: Synthesis of A Modified Oligonucleotide
For simplicity, let’s assume the following structure for the oligonucleotide:
5’ Cy5 – GAAACAGTAAGGCCTTTTTAAGT -[Rox-dT]- ATGGGGAGTAAGTGATCGAACG -Biotin-3’
At first glance, this oligo has three obvious modifications: Cy5 at the 5′ end, Rox-dT internally, and biotin at the 3′ end. The corresponding phosphoramidites for Cy5 and Biotin are available and could easily be incorporated using standard phosphoramidite chemistry. However, there are challenges that complicate this synthesis:
- Availability: Rox-dT is not readily available as a phosphoramidite building block, so an alternative approach is needed.
- Stability: The 5’ Cy5 modification cannot be directly incorporated using Cy5 phosphoramidite because Cy5 is prone to degradation during deprotection and cleavage of longer oligonucleotides, this is also true for a 46mer like in this example.
Taking these limitations into account, the oligonucleotide actually requires five modifications:
- 5’ Amino (e.g., C6) or Alkyne: Used as a precursor for attaching Cy5 via NHS chemistry or click chemistry.
- Internal Amino-C6/C2-dT or Alkyne-dT: Used as a precursor for attaching Rox-dT via NHS chemistry or click chemistry.
- 3’ Biotin: Attached using a suitable solid-phase material for oligo synthesis.
In this case, we need to use different orthogonal strategies for attaching the Cy5 and Rox-dT modifications. Since the amino group can be located both at the 5′-end (terminal) and internally, and it is difficult to distinguish between them in this context, we will apply one dye using click chemistry (or another orthogonal method) and the other using nucleophilic labeling.
Synthesis Strategy
We will begin by synthesizing the following intermediate oligonucleotide (synthesized from 3’ → 5’):
5’ Amino(C6) – GAAACAGTAAGGCCTTTTTAAGT -[C8-Alkyne-dU or EdU] – ATGGGGAGTAAGTGATCGAACG -Biotin-3’
This intermediate oligonucleotide includes an alkyne group (C8-Alkyne-dU or EdU) at the internal position, which will later react with its partner group in the click reaction.
- Purification: After synthesis, we perform one purification step to remove any unreacted or incomplete products.
- Click Reaction: We then perform a click reaction, attaching the Rox-dT modification to the alkyne group. This results in the following modified oligonucleotide:
5’ Amino(C6) – GAAACAGTAAGGCCTTTTTAAGT -[Rox-dT] – ATGGGGAGTAAGTGATCGAACG -Biotin-3’
- Purification: After the click chemistry step, we purify the oligonucleotide again to ensure that the reaction is complete and all byproducts are removed.
- Final Modification: Finally, we use Cy5 NHS ester chemistry to label the terminal Amino (C6) group with Cy5, yielding the final modified oligonucleotide:
5’ Cy5 – GAAACAGTAAGGCCTTTTTAAGT -[Rox-dT] – ATGGGGAGTAAGTGATCGAACG -Biotin-3’
- Final Purification: After this final labeling step, we purify the oligonucleotide one last time to ensure it is pure and ready for use in experiments.
The synthesis of highly modified oligonucleotides, especially those requiring click chemistry, involves a step-by-step approach that combines orthogonal chemistries. By using both phosphoramidite and click chemistry techniques, we can achieve complex modifications that would be difficult or impossible using traditional synthesis methods alone. Key steps include the strategic use of available building blocks, protecting groups, and specialized reagents, followed by careful purification after each modification step. This approach ensures the synthesis of a fully functional, highly modified oligonucleotide.
Other Considerations For Designing Highly Modified Oligonucleotides
The synthesis of highly modified oligonucleotides requires careful planning and consideration of various factors to ensure efficiency, functionality, and reproducibility. Here are some more key design considerations to take into account:
1. Yield and Purification Steps
One of the primary challenges in synthesizing highly modified oligos is balancing yield with the number of purification steps. As more modifications are introduced, the complexity of the synthesis increases, which can lead to lower yields.
- Effect of Purification on Yield: Each purification step removes incomplete or unwanted products, but also results in the loss of some of the desired oligonucleotide. Therefore, excessive purification steps can negatively affect the final yield of the product. This must be carefully optimized to strike a balance between purity and the total amount of the desired oligo. To minimize the impact on yield, it’s important to design the synthesis pathway to limit the number of necessary purification steps.
2. Application-Specific Considerations
Different applications have unique requirements, which may influence the choice of chemical modifications and synthesis methods.
- Therapeutic Applications: For oligos intended for therapeutic use, certain methods may be unsuitable due to potential toxicity or instability. For example, CuAAC (Copper-Catalyzed Azide-Alkyne Cycloaddition), while effective for many types of oligo modifications, is not suitable for therapeutic oligos. This is because the copper catalyst that can introduce toxicity, which could negatively affect the safety and efficacy of the oligo in clinical applications. In comparison, alternative click chemistry methods, such as strain-promoted alkyne-azide cycloaddition (SPAAC), do not require copper and may be a safer option for therapeutic uses.
- Other Applications: For non-therapeutic applications such as fluorescence-based assays, labeling, or diagnostics, CuAAC and similar chemistries are more acceptable and can provide highly efficient conjugation methods. The choice of chemistry should always be based on the specific needs of the application.
3. Reproducibility
- A critical aspect of oligonucleotide synthesis, especially for commercial applications, is ensuring that the oligo can be reliably reproduced across different manufacturers or production batches. This depends on several factors, including the availability of reagents, standardized synthesis methods, and rigorous quality control. Reagents should be available from multiple suppliers to avoid reliance on rare or proprietary sources that may limit reproducibility. Synthesis methods must be well documented and standardized to ensure consistent results across manufacturers. In addition, thorough quality control and testing protocols are essential to ensure that each batch meets the required specifications. This consistency is particularly important for diagnostic and therapeutic applications where reliable product quality is critical.
The synthesis of highly modified oligonucleotides requires careful design to address several considerations. If you have any questions, our team is here to help and find the best solution for your unique oligo design.
We are hiring
Are you ready to combine science with creativity? At Ella Biotech, you’ll join a close-knit team dedicated to advancing the life sciences. You’ll find a vibrant, respectful workplace where teamwork drives innovation and fun is always part of the mix.
Bring your talents to our team where every day is an exciting opportunity to make a difference in biotechnology and advance discovery. Let’s create the building blocks of tomorrow – together!
Contact us
„*“ zeigt erforderliche Felder an
