
Click Chemistry
Click Chemistry
Click chemistry, particularly copper-catalyzed azide-alkyne cycloaddition (CuAAC), has become an invaluable tool for creating diverse modifications in oligonucleotide synthesis. The 2022 Nobel Prize in Chemistry, awarded to Morten Meldal and Barry Sharpless, recognized the significance of CuAAC as a prime example of click chemistry, showcasing its distinctive features of high selectivity, efficiency and yield.
While CuAAC remains the best-known example, two additional types of click reactions—strain-promoted azide-alkyne cycloaddition (SPAAC) and inverse electron-demand Diels-Alder (IEDDA)—also offer valuable alternative options for oligo modifications, broadening the potential for custom, high-quality conjugation services in nucleic acid research and diagnostics.
Azides
In oligonucleotide synthesis, azides are a vital component of click chemistry, serving as the foundation for introducing a wide range of modifications. In both copper-catalyzed and strain-promoted click reactions, the azide group provides a highly selective attachment site for the alkyne counterpart, allowing for efficient and selective connections.
Since azides can react with P(III) in a Staudinger reaction during phosphoramidite based synthesis, they are incorporated post-synthetically—meaning they’re added to the oligo after the primary synthesis usually by attaching them to an existing amino group. This approach allows for precise, targeted modifications that enhance the stability, functionality, and targeting ability of the oligonucleotide, making it highly versatile for applications in research and therapeutic development.
Azides
5' Modifications
Internal Modifications
3' Modifications
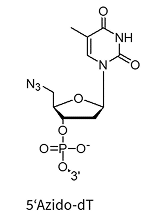
5’Azido-dT*
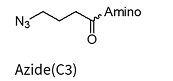
Azide(C3)**
Azido(C3)**-dA/dG/dC/dT
Azide(C3)**
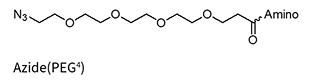
Azide(PEG4)**
Azido(PEG4)**-dA/dG/dC/dT
Azide(PEG4)**
* Can be used for biocompatible click reaction
** Added to the oligo via NHS chemistry and therefore allows for a wide variety of amino linkers
SPAAC Click
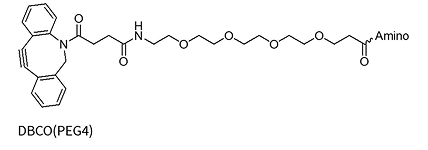
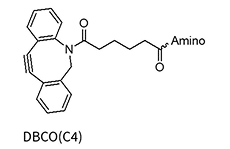
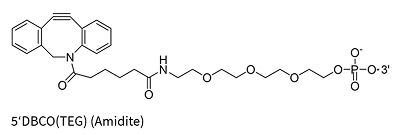
The Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC), often referred to as the „copper-free click,“ is a widely used method for oligonucleotide modification due to its high reactivity under mild, biocompatible conditions without the need for a copper catalyst. This reaction is dependent on the use of strained alkyne rings, such as DBCO (dibenzocyclooctyne) and BCN (bicyclononyne), which are highly reactive with azide-functionalized oligos.
However, the strained alkyne rings are susceptible to oxidation, which restricts their stability and synthetic versatility. Consequently, SPAAC modifications are typically introduced post-synthetically, after the core oligonucleotide has been synthesized. This approach allows for the attachment of complex labels or functional groups, though it requires careful handling to preserve the sensitivity of the strained alkyne, thus supporting the production of high-quality oligo conjugates for advanced research and therapeutic applications.
SPAAC
5' Modifications
Internal Modifications
3' Modifications
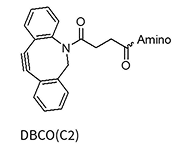
DBCO(C2)*
DBCO(C2)*-dA/dG/dC/dT
DBCO(C2)*
DBCO(PEG4)*
DBCO(PEG4)*-dA/dG/dC/dT
DBCO(PEG4)*
DBCO(TEG)
* Added to the oligo via NHS chemistry and therefore allows for a wide variety of amino linkers
CuAAC Click
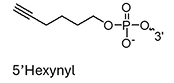
Copper-catalyzed azide-alkyne cycloaddition (CuAAC) is a widely utilized reaction in oligonucleotide synthesis, known for its robustness and efficiency, making it an invaluable asset in the field. Introduced in the early 2000s as a cost-effective alternative to ruthenium-catalyzed methods, CuAAC has unlocked vast potential for oligo modifications, allowing stable and versatile alkyne-functionalized building blocks to be incorporated into synthetic oligonucleotides.
However, the use of copper as a catalyst presents certain challenges, particularly in regard to its cytotoxicity and the risk of damaging sensitive DNA and RNA sequences. To address these challenges, CuAAC is typically reserved for post-synthetic modifications, which allow for controlled conditions that minimize exposure to copper and help maintain oligo integrity. The precise application of CuAAC makes it an invaluable tool for high-specificity conjugation in research and diagnostic oligo applications.
CuAAC Click
5' Modifications
Internal Modifications
3' Modifications
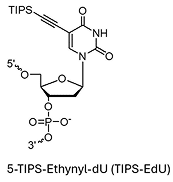
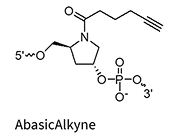
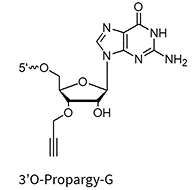
AbasicAlkyne
AbasicAlkyne
AbasicAlkyne
Hexynyl
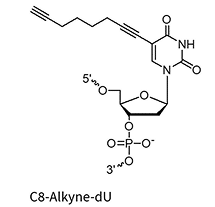
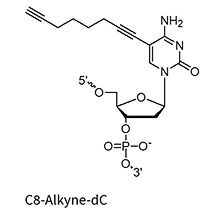
C8-Alkyne-dA/dC/dU*
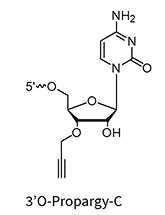
3’Propargyl-5-Methyl-dC**
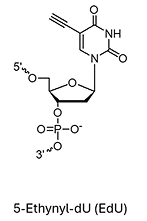
EdU (5-Ethynyl-dU)*
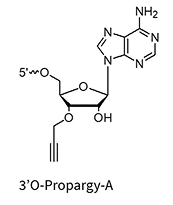
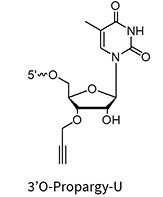
3’Propargyl-A/G/C/U**
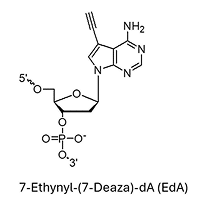
EdA (7-Ethynyl-7-Deaza-dA)*
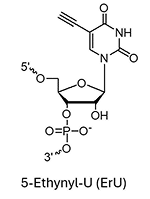
ErU (5-Ethynyl-U)*
TIPS-EdU (5-TIPS-Ethynyl-dU)*
* Under license from baseclick GmbH
** Can be used for biocompatible click reaction
Tetrazine
Tetrazine-based click chemistry, specifically through inverse electron demand Diels–Alder (IEDDA) reactions, offers a unique and highly effective approach for oligonucleotide modification. This reaction allows for highly selective and efficient labeling under mild, biocompatible conditions, as it does not require a catalyst. It is particularly useful for labeling with tetrazines, which react with strained alkenes, such as trans-cyclooctenes, without requiring a catalyst.
Tetrazine conjugation is an invaluable tool for applications where copper-free and strain-promoted methods are preferred, as it avoids cytotoxicity and preserves nucleic acid integrity. Due to its rapid rate of reaction and stability, tetrazine click chemistry is frequently used in post-synthetic modifications of oligonucleotides. This enables the precise attachment of fluorophores, affinity tags, or other functional groups. This approach is ideal for the creation of multifunctional oligonucleotides for advanced imaging, diagnostics, and therapeutic research.
Tetrazine
5' Modifications
Internal Modifications
3' Modifications
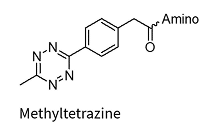
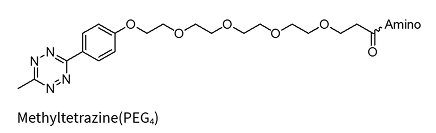
Methyltetrazine*
Methyltetrazine*-dA/dG/dC/dT
Methyltetrazine*
Methyltetrazine(PEG4)*
Methyltetrazine-PEG4*-dA/dG/dC/dT
Methyltetrazine(PEG4)*
* Added to the oligo via NHS chemistry and therefore allows for a wide variety of amino linkers
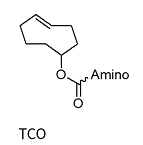
IEDDA: trans-Cyclooctenen (TCO)
Trans-cyclooctene (TCO) is a useful modification in oligonucleotide chemistry, particularly for facilitating bioorthogonal conjugation via inverse electron demand Diels-Alder (IEDDA) reactions. As a strained alkene, TCO reacts extremely rapidly and selectively with tetrazines, allowing for efficient, copper-free labeling of oligonucleotides that are also biocompatible.
While TCO itself can be incorporated during or after oligonucleotide synthesis, the actual labeling with tetrazine-functionalized molecules typically occurs post-synthetically. This approach offers flexibility and allows site-specific labeling and attachment of various functional groups such as fluorescent probes, affinity tags, or drug molecules.
TCO
5' Modifications
Internal Modifications
3' Modifications
TCO*
TCO*-dA/dG/dC/dT
TCO*
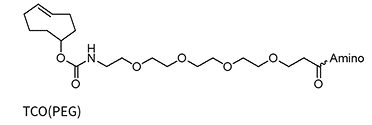
TCO(PEG4)*
TCO(PEG4)*-dA/dG/dC/dT
TCO(PEG4)*
* Added to the oligo via NHS chemistry and therefore allows for a wide variety of amino linkers
We are hiring
Are you ready to combine science with creativity? At Ella Biotech, you’ll join a close-knit team dedicated to advancing the life sciences. You’ll find a vibrant, respectful workplace where teamwork drives innovation and fun is always part of the mix.
Bring your talents to our team where every day is an exciting opportunity to make a difference in biotechnology and advance discovery. Let’s create the building blocks of tomorrow – together!
Contact us
„*“ zeigt erforderliche Felder an
